Elements with four outermost electrons such as carbon find it easier to share electrons than attempt to gain or lose four electrons. The bond formed by sharing of electrons between the two atoms is called covalent bond.
Q. What will happen if carbon lose 4 electrons?
if it loses 4 electrons, it would require a large amount of energy to remove 4 electrons leaving behind a carbon cation with six protons in its nucleus holding on to just 2 electrons. carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements.
Table of Contents
- Q. What will happen if carbon lose 4 electrons?
- Q. Does carbon gain or lose electrons to become stable?
- Q. Why C4+ and C4 is not formed?
- Q. Why carbon Cannot Form C 4 or C 4 ion?
- Q. Why C4 is not possible?
- Q. Why C4 ions are not formed?
- Q. Why does carbon not form C4+?
- Q. What is anion mode?
- Q. How do you determine the size of an anion?
- Q. How do you determine the size of a cation and anion?
- Q. What determines the size of a cation?
Q. Does carbon gain or lose electrons to become stable?
Carbon would need to either lose four electrons or gain four electrons in order to have a full valence shell. Atoms will form as many covalent bonds as it takes to fill their valence shell. This means that carbon, our previous example, will need to form four covalent bonds in order to fill its outermost shell.
Q. Why C4+ and C4 is not formed?
a Carbon cannot form C4+ cation because of removal of 4 elections from a carbon atom would require a large amount of energy. b Carbon cannot form C4- anion because it would be difficult for the nucleus with 6 protons to hold on to 10 electrons. c Hence carbon atoms share electrons forming covalent compounds.
Q. Why carbon Cannot Form C 4 or C 4 ion?
To get the octet in its outer shell it has to gain 4 more electrons to form C4- ion. the electronegativity of carbon is only 2.5 and its nucleus has only 6 protons. therefore its difficult for a nucleus with 6 protons to hold 10 electrons. Hence carbon cannot form C4- ions so easily.
Q. Why C4 is not possible?
it highly unstable. Carbon is unable to form C4- anion as its nucleus with six protons will not be able to hold ten electrons. (i) Covalent compounds are bad conductors of electricity due to lack of free electrons.
Q. Why C4 ions are not formed?
Carbon cannot add up 4 more electrons in its outer orbital that is 2p orbital. Hence carbon cannot form C4− ions so easily. Carbon has to satisfy tetravalency, by sharing electrons with the other atoms. so it has to form 4 covalent bonds either with its own atoms or atoms of other elements.
Q. Why does carbon not form C4+?
a Carbon cannot form C4+ cation because of removal of 4 elections from a carbon atom would require a large amount of energy. d Covalent compounds do not form ions/ charged particles and therefore do not conduct electricity. e Inter molecular forces of attraction are weak hence low melting and boiling points.
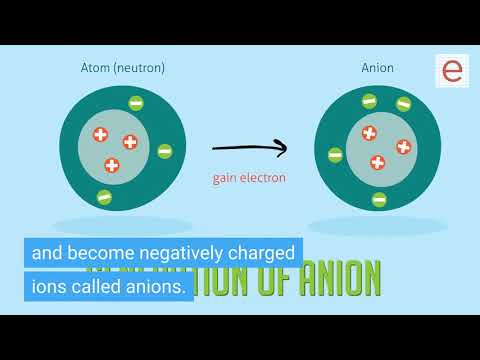
Q. What is anion mode?
Anion Button turns the ANION function ON or OFF. The anion function creates negative ions, which cause floating particulates (dust, pet dander, allergens) in the air to cling together and drop to the floor.
Q. How do you determine the size of an anion?
In general, ionic radius decreases with increasing positive charge. As the charge on the ion becomes more positive, there are fewer electrons. The ion has a smaller radius. In general, ionic radius increases with increasing negative charge.
Q. How do you determine the size of a cation and anion?
- Cations are therefore smaller than the parent atom.
- Anions are therefore larger than the parent atom.
- For ions of the same charge (e.g. in the same group) the size increases as we go down a group in the periodic table.
Q. What determines the size of a cation?
1 Answer. When atoms gain or lose electrons, the atom becomes an ion. Anions of atoms are larger in size from their parent atom and cations are smaller..