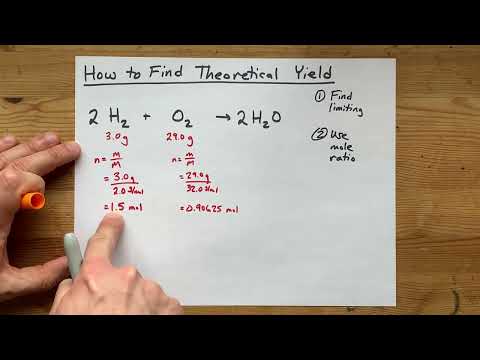
Usually, percent yield is lower than 100% because the actual yield is often less than the theoretical value. Reasons for this can include incomplete or competing reactions and loss of sample during recovery. This can happen when other reactions were occurring that also formed the product.
Q. Does temperature affect catalyst?
Usually reactions speed up with increasing temperature (“100C rise doubles rate”). Physical state of reactants. Powders react faster than blocks – greater surface area and since the reaction occurs at the surface we get a faster rate. The presence (and concentration/physical form) of a catalyst (or inhibitor).
Table of Contents
- Q. Does temperature affect catalyst?
- Q. What factors affect percentage yield?
- Q. Why is it impossible to get 100% yield?
- Q. What increases yield?
- Q. Why is the theoretical yield never obtained?
- Q. What is a good percent yield?
- Q. Why can the actual yield be less than the theoretical yield?
- Q. Can a reaction have 110 actual yield?
- Q. Is actual or theoretical yield bigger?
- Q. How do you find actual yield with only theoretical yield?
- Q. What is the difference between actual yield and theoretical yield?
- Q. How do you calculate the overall yield?
- Q. Do you reduce mole ratios?
- Q. What is the mole ratio of NaOH to Al OH 3?
- Q. Why is mole ratio important?
- Q. What is the mole ratio of pcl3 to pcl5?
Q. What factors affect percentage yield?
The yield and rate of a chemical reaction depend on conditions such as temperature and pressure. In industry, chemical engineers design processes that maximise the yield and the rate at which the product is produced. They also aim to reduce waste and energy costs at all stages of the process.
Q. Why is it impossible to get 100% yield?
The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. However, percent yields greater than 100% are possible if the measured product of the reaction contains impurities that cause its mass to be greater than it actually would be if the product was pure.
Q. What increases yield?
Le Châtelier’s Principle states that a change in pressure, temperature, or concentration will push the equilibrium to one side of the chemical equation. So, if you manipulate the conditions to favour the product side, you increase the yield.
Q. Why is the theoretical yield never obtained?
Reasons for not achieving the theoretical yield. Possible reasons for not achieving the theoretical yield. Reaction may stop short of completion so that reactants remain unreacted. There may be competing reactions that give other products and therefore reduce the yield of the desired one.
Q. What is a good percent yield?
According to the 1996 edition of Vogel’s Textbook , yields close to 100% are called quantitative, yields above 90% are called excellent, yields above 80% are very good, yields above 70% are good, yields above 50% are fair, and yields below 40% are called poor.
Q. Why can the actual yield be less than the theoretical yield?
Why Is Actual Yield Different from Theoretical Yield? Usually, the actual yield is lower than the theoretical yield because few reactions truly proceed to completion (i.e., aren’t 100% efficient) or because not all of the product in a reaction is recovered.
Q. Can a reaction have 110 actual yield?
This percent yield is just a concept to measure the extent of a chemical reaction because in actual situations, reactions are rarely proceeding to completion. Thus, to put it simply, a chemical reaction can never have 110% actual yield, or anything beyond 100% for that matter.
Q. Is actual or theoretical yield bigger?
An actual yield is the mass of a product actually obtained from the reaction. It is usually less than the theoretical yield.
Q. How do you find actual yield with only theoretical yield?
The actual yield is expressed as a percentage of the theoretical yield. This is called the percent yield. To find the actual yield, simply multiply the percentage and theoretical yield together.
Q. What is the difference between actual yield and theoretical yield?
Theoretical yield is what you calculate the yield will be using the balanced chemical reaction. Actual yield is what you actually get in a chemical reaction.
Q. How do you calculate the overall yield?
Note that if a synthesis is a linear multistep process, then the overall yield is the product of the yields of each step. So for example, if a synthesis has two steps, each of yield 50% then the overall yield is 50% x 50% = 25%.
Q. Do you reduce mole ratios?
The molar ratio can be constructed using any two compounds in the reaction, be they reactants or products. Write the molar ratios for (a) O2 to SO3 and (b) SO2 to SO3. Note that both ratios can be reduced. Eventually, ratios like the above will be used in calculations.
Q. What is the mole ratio of NaOH to Al OH 3?
3 to 1
Q. Why is mole ratio important?
Mole ratios are important because mole ratios allow you change moles of a substance to moles of another substance. The mole ratios come from the chemical formula or equation.
Q. What is the mole ratio of pcl3 to pcl5?
Answer : The mole ratio of to is 1 : 1 ratio.