At low temperatures, attractions between gas particles cause the particles to collide less often with the container walls, resulting in a pressure lower than the ideal gas value.
Q. Which temperature scale is used in gas laws?
Kelvin scale
Table of Contents
- Q. Which temperature scale is used in gas laws?
- Q. Why do gases fail to obey the general gas equation at high pressure and low temperature?
- Q. Why real gases do not follow the ideal gas equation?
- Q. Is the ideal gas law valid for every gas?
- Q. Which gas behaves most like an ideal gas?
- Q. Is methane an ideal gas?
- Q. Is h2 more ideal than he?
- Q. Is xenon an ideal gas?
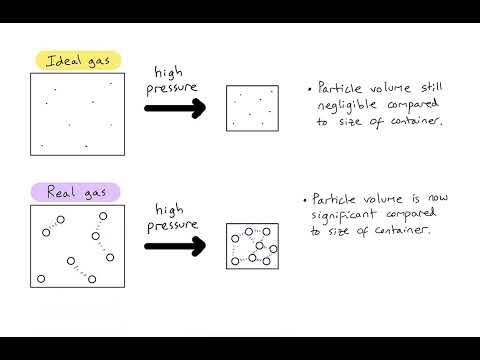
Q. Why do gases fail to obey the general gas equation at high pressure and low temperature?
Answer: At low temperatures and high pressure, the inter-molecular interactions of the gases become significant, as molecules are brought closer. Also, the volume of the gases takes a finite value as they are compressed under high pressure and low temperature. Therefore, the gas laws become invalid at low temp.
Q. Why real gases do not follow the ideal gas equation?
1: Real Gases Do Not Obey the Ideal Gas Law, Especially at High Pressures. (a) In these plots of PV/nRT versus P at 273 K for several common gases, there are large negative deviations observed for C2H4 and CO2 because they liquefy at relatively low pressures. 2: The Effect of Temperature on the Behavior of Real Gases.
Q. Is the ideal gas law valid for every gas?
The ideal gas equation is equally valid for any gas, whereas the van der Waals equation contains a pair of constants (a and b) that change from gas to gas. At normal temperatures and pressures, the ideal gas and van der Waals equations give essentially the same results.
Q. Which gas behaves most like an ideal gas?
helium
Q. Is methane an ideal gas?
Methane (CH4) behaves as an ideal gas under standard temperature and pressure conditions.
Q. Is h2 more ideal than he?
Interestingly, at 250 K hydrogen is more ideal than helium (if only slightly so), if the vdW prediction is to be trusted. An interesting aside is that helium has the lowest values of critical temperature Tc and critical pressure Pc.
Q. Is xenon an ideal gas?
Xenon is an ideal gas for this model with its cardiovascular stability [47] and myocardial protection property [48], as well as its rapid induction rate through blood–brain-barrier [14, 49].