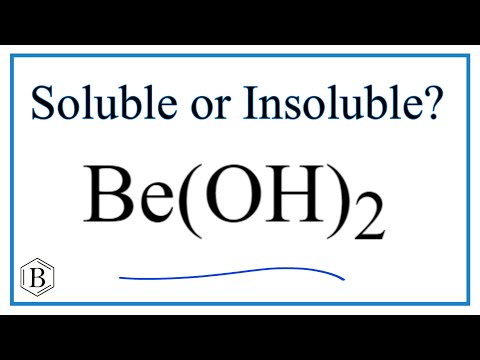
Be(OH)2 and Mg(OH)2 are almost insoluble, Ca(OH)2 is sparingly soluble while Sr(OH)2 and Ba(OH)2 are fairly soluble. This is due to increase in size of the cation and decrease in their lattice energies on moving down the group.
Q. Why is the charge of MgCl2 +2?
Explanation: Salts are electrically neutral. In magnesium chloride or calcium chloride or ferrous chloride, the charge on the metal centre is equally balanced by the chlorides. Cl− bears a single negative charge, and thus 2 equiv of chloride are required to give a salt.
Table of Contents
Q. Why MgCl2 is soluble in water?
Answer. Mgcl2 is an ionic compound which can dissolve in water as a high dielectric constant force due to which it weakens the attraction between the ions. For ex sodium chloride also has dielectric constant due to which it can soluble.
Q. Is BA OH 2 soluble or insoluble in water?
Ba(OH)2 is soluble in water and gives a alkaline solution. The maximum molarity at 25 C is 0.15 mole/liter.
Q. Is Fe OH 2 a base or acid?
pH of Common Acids and Bases
| Base | Name | 1 mM |
|---|---|---|
| NH4OH | ammonium hydroxide (NH3:H2O) | 10.09 |
| Mg(OH)2 | magnesium hydroxide (MgO:H2O) | 10.40 |
| CaCO3 | calcium carbonate (calcite) | 9.91 |
| Fe(OH)2 | iron(II) hydroxide (ferrous hydroxide) | 9.45 |
Q. Why BaSO4 is sparingly soluble?
Answer. BaSO₄ is sparingly soluble in water due to its more lattice energy. This leads to the increase in the hydration energy and decrease in lattice energy. In case of BaSO₄ hydration energy decreases and lattice energy increases.
Q. Is Na3PO4 a solid?
Sodium Phosphate, Tribasic is an odorless, white, crystalline (sand-like) solid.
Q. Are all nitrates soluble in water?
All nitrates are soluble. All acetates (ethanoates) are soluble. EXCEPT those of ammonium (NH4+) and Alkali metal (Group 1, or, Group IA) cations.
Q. Is feso4 soluble in water?
Ferrous sulfate heptahydrate is freely soluble in water; very soluble in boiling water; practically insoluble in ethanol (~750 g/l) TS.