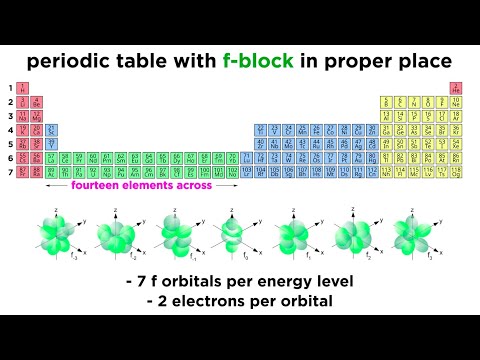
Introduction to Lanthanides The f-block elements in the periodic table appear in two series characterized by the filling of the 4f and 5f orbitals. The first series contain the fourteen elements cerium to lutecium (elements 58 through 71) and are called lanthanides because they appear after lanthanum.
Q. How many electrons are in the lanthanide series?
The lanthanide series includes elements from number 58 to 71, which is 14 elements. The f sub-level contains seven orbitals and each orbital will hold two electrons. Therefore, it is possible to place 14 electrons in the 4f sub-level.
Table of Contents
- Q. How many electrons are in the lanthanide series?
- Q. Do lanthanides have valence electrons?
- Q. What electrons do lanthanides lose?
- Q. What is the outer electronic configuration of lanthanides?
- Q. What do lanthanides have in common?
- Q. What are the similarities and differences between lanthanides and actinides?
- Q. What are the two similarities between lanthanides and actinides?
- Q. What are the four similarities lanthanides and Actinoids?
- Q. What do lanthanides and actinides have in common?
- Q. Why are lanthanides and actinides in periods 6 and 7?
- Q. What is the meaning of actinides?
Q. Do lanthanides have valence electrons?
Since the lanthanide metals (except europium and ytterbium) have three valence electrons, in order to form dihalides in whcih lanthanides are in the divalent state there must be a change from the trivalent state to the divalent by removing an electron from the valence band and putting it into the 4f level.
Q. What electrons do lanthanides lose?
The chemistry of the lanthanides is dominated, in fact, by the +3 oxidation state, in which the two 6s electrons and (usually) one 4f electron are lost to form tripositive (+3) ions. Generally speaking, this is the most stable oxidation state for the lanthanide elements.
Q. What is the outer electronic configuration of lanthanides?
Complete answer: The general electronic configuration of lanthanides is (n−2)f1−14(n−1)d0−1ns2 . But the penultimate subshell also contains (n−1)s2 and (n−1)p6 electrons. Hence, the general electronic configuration of lanthanides will be (n−2)f1−14(n−1)s2(n−1)p6(n−1)d0−1ns2.
Q. What do lanthanides have in common?
Lanthanides share the following common properties:
- Silvery-white metals that tarnish when exposed to air, forming their oxides.
- Relatively soft metals.
- Moving from left to right across the period (increasing atomic number), the radius of each lanthanide 3+ ion steadily decreases.
- High melting points and boiling points.
Q. What are the similarities and differences between lanthanides and actinides?
> Similarities: One of the major similarities between lanthanide and actinide elements in the periodic table is that all of them are f-block elements….Complete answer:
| Lanthanides | Actinides |
|---|---|
| All except Promethium (Pm) are not radioactive. | All are radioactive |
| Most form colourless compounds | Most form coloured compounds |
Q. What are the two similarities between lanthanides and actinides?
1. These both series elements mostly show an oxidation number +3 and elements are electropositive and very reactive. 2. Lanthanoids and actinoids show contractions in their radii and both show magnetic properties.
Q. What are the four similarities lanthanides and Actinoids?
Both have a prominent oxidation state of +3. They are involved in the filling of (n-2) f orbitals. They are highly electropositive and very reactive in nature. With an increase in atomic number, there is a decrease in atomic and ionic size.
Q. What do lanthanides and actinides have in common?
All the lanthanide elements exhibit the oxidation state +3. Actinides are typical metals. All of them are soft, have a silvery color (but tarnish in air), and have relatively high density and plasticity. Unlike the lanthanides, most elements of the actinide series have the same properties as the d block.
Q. Why are lanthanides and actinides in periods 6 and 7?
The reason why Lanthanides and Actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. The lanthanides include elements 58 to 71 (fill out the 4f subshell) and the actinides include elements 89 to 103 (fill out the 5f subshell).
Q. What is the meaning of actinides?
noun. 1. Any of a series of chemically similar metallic elements with atomic numbers ranging from 89 (actinium) to 103 (lawrencium). All of these elements are radioactive, and two of the elements, uranium and plutonium, are used to generate nuclear energy.