philosopher Democritus
Q. How many of each of the subatomic particles does an atom have?
three subatomic particles
Table of Contents
- Q. How many of each of the subatomic particles does an atom have?
- Q. What are the locations of the 3 subatomic particles?
- Q. How many subatomic particles are in a proton?
- Q. What are the 3 subatomic particles of an atom and their charges?
- Q. Why was Rutherford’s model important?
- Q. What are the four principles of Bohr’s model?
- Q. Why is the Bohr model still used today?
- Q. Is Bohr’s model still relevant?
- Q. Why is Bohr’s model better than Rutherford?
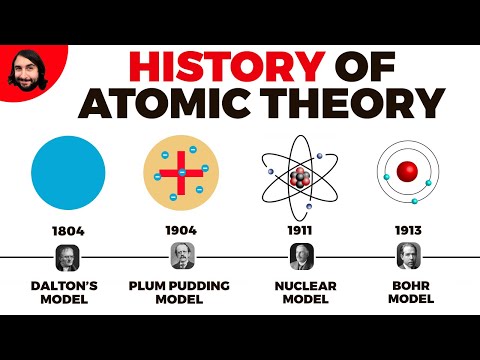
- Q. Which atomic model is missing from this set?
- Q. What are the similarities and differences between Thomson’s and Rutherford’s atomic models?
- Q. How did JJ Thomson determine the charge of an electron?
- Q. How did JJ Thomson discover the E M ratio of cathode rays?
Q. What are the locations of the 3 subatomic particles?
The Three Major Subatomic Particles
| Name | Symbol | Location |
|---|---|---|
| Proton | P+ | Nucleus |
| Neutron | n0 | Nucleus |
| Electron | e– | Outside Nucleus |
Q. How many subatomic particles are in a proton?
A proton is made of two up quarks and one down quark, while the neutron is made of two down quarks and one up quark. These commonly bind together into an atomic nucleus, e.g. a helium-4 nucleus is composed of two protons and two neutrons.
Q. What are the 3 subatomic particles of an atom and their charges?
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. An easy way to remember this is to remember that both proton and positive start with the letter “P.” Neutrons have no electrical charge.
Q. Why was Rutherford’s model important?
Most important, he postulated the nuclear structure of the atom: experiments done in Rutherford’s laboratory showed that when alpha particles are fired into gas atoms, a few are violently deflected, which implies a dense, positively charged central region containing most of the atomic mass.
Q. What are the four principles of Bohr’s model?
The Bohr model can be summarized by the following four principles: Electrons occupy only certain orbits around the nucleus. Those orbits are stable and are called “stationary” orbits. Each orbit has an energy associated with it.
Q. Why is the Bohr model still used today?
Today, we know that the Bohr Model has some inaccuracies, but it’s still used because of its simple approach to atomic theory. The Bohr model was also the first atomic model to incorporate quantum theory, meaning that it’s the predecessor of today’s more accurate quantum-mechanical models.
Q. Is Bohr’s model still relevant?
Bohr’s atomic model was utterly revolutionary when it was presented in 1913 but, although it is still taught in schools, it became obsolete decades ago. Most schools still teach the atomic model, in which electrons orbit around the nucleus like the planets do around the sun.
Q. Why is Bohr’s model better than Rutherford?
Rutherford randomly placed the negative electrons outside the nucleus. Bohr’s improvement of the Rutherford model was that Bohr placed the electrons in distinct energy levels. Bohr thought that electrons orbited the nucleus in quantised orbits.
Q. Which atomic model is missing from this set?
Dalton model
Q. What are the similarities and differences between Thomson’s and Rutherford’s atomic models?
Similarities between Thomson and Rutherford’s atomic model is that there are negative and positive charges in an atom. Another similarity is in the shape,Thomson describes a sphere and Rutherford describes orbiting around the nucleus, which is sphere shaped.
Q. How did JJ Thomson determine the charge of an electron?
Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson’s plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged “soup.”
Q. How did JJ Thomson discover the E M ratio of cathode rays?
The Discovery of the Electron (J. J. Thomson) By balancing the effect of a magnetic field on a cathode-ray beam with an electric field, Thomson was able to show that cathode “rays” are actually composed of particles. This experiment also provided an estimate of the ratio of the charge to the mass of these particles.