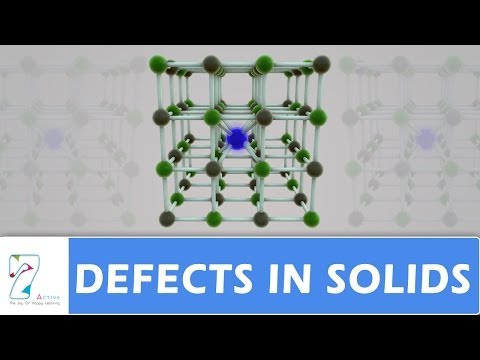
Examples. This type of defect is typically observed in highly ionic compounds, highly coordinated compounds, and where there is only a small difference in sizes of cations and anions of which the compound lattice is composed. Typical salts where Schottky disorder is observed are NaCl, KCl, KBr, CsCl and AgBr.
Q. Why TiO2 is N type semiconductor?
The oxygen vacancies act as electron donors, thus TiO2-x is an n-type semiconductor, in contrast with p-type semiconductors which contain electron acceptors and where the charge carriers are holes rather than electrons [23].
Table of Contents
- Q. Why TiO2 is N type semiconductor?
- Q. What do you mean by point defect in a crystal?
- Q. Does AgCl show Frenkel defect?
- Q. What happens when CdCl2 is doped with AgCl?
- Q. What happens when CdCl2 is added to NaCl?
- Q. When AgCl crystal is doped with then it produces a?
- Q. What happens when NaCl is doped with SrCl2?
- Q. What happens when small amount of SrCl2 or Agcl is added to molten NaCl?
- Q. What happens when NaCl is doped with AlCl3?
Q. What do you mean by point defect in a crystal?
Point defects are caused by missing or misplaced ion or atom in the crystal lattice i.e., deviation from regular arrangement of ions/ particles in the crystal. Formula of a compound is same as the formula of unit cell.
Q. Does AgCl show Frenkel defect?
AgCl shows Frenkel defect while NaCl does not. Frenkel defect is shown by those in which there is large difference in the size of cations and anions. Hence, Frenkel defect is shown by AgCl due to small size of Ag+ ion but not by NaCl because alkali metal ions can not fit into interstital sites.
Q. What happens when CdCl2 is doped with AgCl?
When AgCl is doped with CdCl2, cationic vacancy defect is produced. – When AgCl is doped with CdCl2, each Cd++ ion replaces two Ag+ ions to balance the charge. – As a result, overall 1 cationic vacancy is created per CdCl2 molecule added. – This is called vacancy defect.
Q. What happens when CdCl2 is added to NaCl?
When sodium chloride is doped with cadmium chloride, the type of defect is called an IMPURITY DEFECT. Here, to maintain electrical neutrality, each Cd2+ ion replaces 2 Na+ ions. Thus, the density of the crystal decreases due to cationic vacancies produced. hence the mechanical strength of crystal also decreases.
Q. When AgCl crystal is doped with then it produces a?
When AgCl is doped with CdCl2 a cation vacancy defect is created, i.e., an Ag+ ion from the lattice is absent from its position due to presence of adjacent Cd2+ ion.
Q. What happens when NaCl is doped with SrCl2?
Answer Expert Verified When the NaCl is doped with Sr+2 ,it forms solid solution. Few Na+1 gets replaced by few Sr+2. Hence the doping i.e adding of impurities of SrCl2 changes the structure of NaCl. thanks!
Q. What happens when small amount of SrCl2 or Agcl is added to molten NaCl?
Answer: When molten NaCl (Na+ is monovalent) containing a little amount of SrCl2 (Sr2+ is divalent) as impurity is crystallised, some of the sites of Na+ ions are occupied by Sr2+. Each Sr2+ replaces two Na+ ions. The cationic vacancies thus produced are equal to the number of Sr2+ ions.
Q. What happens when NaCl is doped with AlCl3?
If NaCl is doped with 10-4 mol% of AlCl3, the concentration of cation vacancies will be (NA = 6.02 x 1023) (1) 6.02 x 1017 mol-1. (2) 2 x 6.02 x 1017 mol-1. (3) 3 × 6.02 x 1017 mol-1.