CCl4 is a non-polar molecule as the individual C−Cl bonds are polar but due to the tetrahedral shape of CCl4, the bond polarity cancels out and thus the net dipole moment is zero.
Q. How do you classify a molecule polar or nonpolar?
(If the difference in electronegativity for the atoms in a bond is greater than 0.4, we consider the bond polar. If the difference in electronegativity is less than 0.4, the bond is essentially nonpolar.) If there are no polar bonds, the molecule is nonpolar. If the molecule has polar bonds, move on to Step 3.
Table of Contents
- Q. How do you classify a molecule polar or nonpolar?
- Q. What is a nonpolar covalent bond example?
- Q. What is the formula for nonpolar covalent compound?
- Q. What is a nonpolar compound?
- Q. Why is it important to classify a compound as polar or nonpolar?
- Q. Which of the following is nonpolar?
- Q. What is polar and nonpolar compound?
- Q. What is polar molecule give example?
- Q. What is difference between polar and nonpolar molecule?
- Q. What are three types of polarity?
- Q. How do you describe polarity?
- Q. What is polarity measured in?
- Q. Does size affect polarity?
- Q. What is the SI unit of dipole moment?
- Q. What is the symbol of dipole moment?
- Q. What is a dipole moment vector?
- Q. What are the applications of dipole moment?
- Q. How do you do a dipole symbol?
- Q. How can you determine which way to draw a bond dipole?
- Q. How do you find the dipole moment of HCl?
Q. What is a nonpolar covalent bond example?
Nonpolar covalent bonds are a type of bond that occurs when two atoms share a pair of electrons with each other. These shared electrons glue two or more atoms together to form a molecule. An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they equally share the electrons.
Q. What is the formula for nonpolar covalent compound?
Carbon forms a double bond (C=O) with each oxygen atom, and the electrons are not shared equally between the two atoms because of electronegativity difference….Examples of Nonpolar Covalent Molecules with Polar Covalent Bonds.
| Compound Name | Molecular formula | Polar covalent bond |
|---|---|---|
| Silicon dioxide | SiO2 | Si=O |
| Methane | CH4 | C-H |
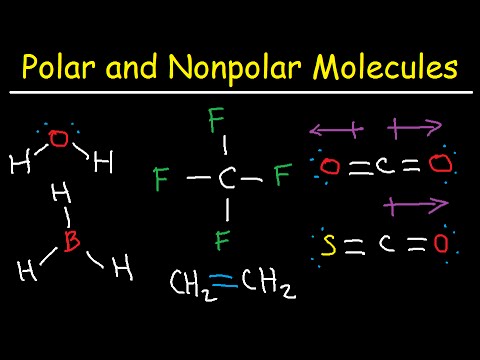
Q. What is a nonpolar compound?
noun, plural: nonpolar compounds. A compound comprised of molecules linked through chemical bonds arranged in such a way that the distribution of charges are symmetrical. Supplement. Nonpolar compounds do not exhibit polarity.
Q. Why is it important to classify a compound as polar or nonpolar?
Why is it important to classify a compound as polar or nonpolar? Because we can know if it’s a solvent or not, so we can know if it can be dissolved in the bloodstream.
Q. Which of the following is nonpolar?
CO2 Is a linear symmetrical structure, hence the summation of all the vectors is zero. So this molecule is nonpolar. So, the correct answer is “Option B”.
Q. What is polar and nonpolar compound?
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
Q. What is polar molecule give example?
Examples of Polar Molecules Ethanol is polar because the oxygen atoms attract electrons because of their higher electronegativity than other atoms in the molecule. Thus the -OH group in ethanol has a slight negative charge. Ammonia (NH3) is polar. Sulfur dioxide (SO2) is polar.
Q. What is difference between polar and nonpolar molecule?
Chemical bonds exists as polar covalent bonds and nonpolar covalent bonds….Nonpolar:
| Difference between Polar and Nonpolar | |
|---|---|
| POLAR | NONPOLAR |
| At Least one polar covalent is present in all polar molecules | Nonpolar covalent is not present in all nonpolar molecules |
| Charge separation | No charge separation |
| Dipole moment | No dipole moment |
Q. What are three types of polarity?
It describes the nature of the international system at any given period of time. One generally distinguishes three types of systems: unipolarity, bipolarity, and multipolarity for three or more centers of power.
Q. How do you describe polarity?
In chemistry, polarity refers to the way in which atoms bond with each other. A polar molecule arises when one of the atoms exerts a stronger attractive force on the electrons in the bond. The electrons get drawn more towards that atom, so that the molecule exhibits a slight charge imbalance.
Q. What is polarity measured in?
dipole moment
Q. Does size affect polarity?
The shape of the molecule will determine the direction of each of the individual bond dipoles, and thus, will always play a role in determining the polarity of the molecule as a whole.
Q. What is the SI unit of dipole moment?
ampere second
Q. What is the symbol of dipole moment?
μ
Q. What is a dipole moment vector?
The dipole moment of two equal but opposite charges (q+, q−) is defined as the product of charges and the distance separating them; thus it is a vector quantity where the magnitude of the dipole moment vector is qr and the direction is from the negative charge to the positive.
Q. What are the applications of dipole moment?
(i) Ionic character can be calculated using the value of dipole moment. (ii) Geometry of the molecule can be predicted using the dipole moment. (iii) Dipole moment is helpful in predicting nature of the molecule.
Q. How do you do a dipole symbol?
The dipole moment of a molecule, being a vector, has a direction, which is shown using the following symbol. The arrowhead of the symbol is pointed toward the negative pole and the plus sign toward the positive pole. eg: A molecule may not have a dipole moment despite containing bonds that do.
Q. How can you determine which way to draw a bond dipole?
A bond dipole is represented an arrow. The direction of the arrow always points the the negative end of the bond. A “+” is placed at the tail of the arrow to indicate the positive end of the bond. Bond dipoles are vectors.
Q. How do you find the dipole moment of HCl?
Dipole moment can be calculated as the product of the charge (abbreviated Q) times the distance (abbreviated r) between the charges. Example: The dipole moment of HCl is 1.03 D, and the bond length is 127 pm. What is the percent ionic character of the HCl bond?