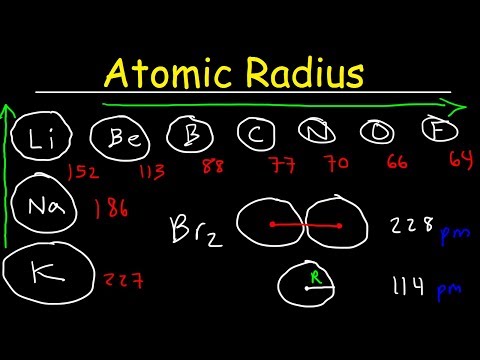
(B) Trends in the Atomic Radius of Group 17 (VIIA, Halogens) Elements
Q. Which is the largest atom in Group 4a 14?
lead
Table of Contents
- Q. Which is the largest atom in Group 4a 14?
- Q. What is Group 4A called?
- Q. What is Group 5A called?
- Q. What is Group 6A called?
- Q. What is the most electronegative element in Group 5A?
- Q. Which group has the highest electronegativity?
- Q. Which period has the highest electronegativity?
- Q. Who has the highest electronegativity?
- Q. Which is the most electropositive element?
- Q. Which is more electropositive Na or K?
- Q. Which is more electropositive Na or H?
- Q. Why magnesium is highly Electropositive?
- Q. Are all metals Electropositive?
- Q. Is mg more Electropositive?
- Q. Are acids Electropositive?
- Q. Is more electropositive than Aluminium?
- Q. Which has more metallic character beryllium or Aluminium?
- Q. Which group has more metallic character?
Q. What is Group 4A called?
Group 4A (or IVA) of the periodic table includes the nonmetal carbon (C), the metalloids silicon (Si) and germanium (Ge), the metals tin (Sn) and lead (Pb), and the yet-unnamed artificially-produced element ununquadium (Uuq).
| Element | Atomic Number (Z) | Trend |
|---|---|---|
| fluorine | 9 | (smallest) ↓ |
| chlorine | 17 | ↓ |
| bromine | 35 | ↓ |
| iodine | 53 | ↓ (largest) |
Q. What is Group 5A called?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
Q. What is Group 6A called?
Group 6A (or VIA) of the periodic table are the chalcogens: the nonmetals oxygen (O), sulfur (S), and selenium (Se), the metalloid tellurium (Te), and the metal polonium (Po). The name “chalcogen” means “ore former,” derived from the Greek words chalcos (“ore”) and -gen (“formation”).
Q. What is the most electronegative element in Group 5A?
20.7 Group 7A(17): The Halogens This group contains nonmetals that are generally very reactive and each of them can form compounds with most elements. At the top of the group is fluorine, which is the most electronegative and most reactive of all nonmetals.
Q. Which group has the highest electronegativity?
fluorine
Q. Which period has the highest electronegativity?
In Period 3, sodium with 11 protons is the least electronegative element, and chlorine with 17 protons is the most electronegative element. You might expect argon (with 18 electrons) to be the most electronegative element in Period 3.
Q. Who has the highest electronegativity?
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. (Helium, neon, and argon are not listed in the Pauling electronegativity scale, although in the Allred-Rochow scale, helium has the highest electronegativity.)
Q. Which is the most electropositive element?
Cesium
Q. Which is more electropositive Na or K?
Potassium (K) is more electropositive than sodium (Na). So potassium is below sodium with greater atomic number.
Q. Which is more electropositive Na or H?
Sodium is more electropositive than hydrogen although they are in the same group.
Q. Why magnesium is highly Electropositive?
Because it has the ability to lose electrons. It can remove two electrons to form stable Mg2+.
Q. Are all metals Electropositive?
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Thus metals are electropositive elements with relatively low ionization energies.
Q. Is mg more Electropositive?
– So, we can see that the barium has the largest atomic no. and has maximum no. of shells in the atom. – So, among Be, Mg, Ca and Ba, Barium has the largest size due to which it can easily lose the outermost electron from the shell and will be considered as the most electropositive element.
Q. Are acids Electropositive?
In general, the electropositive character of the oxide’s central atom will determine whether the oxide will be acidic or basic. The more electropositive the central atom, the more basic the oxide. We define non-metal oxide acidity in terms of the acidic solutions formed in reactions with water.
Q. Is more electropositive than Aluminium?
Thus Beryllium (Be) is the Most electropositive element among them. Aluminium lies below the Boron (B) in periodic table thus it has more electropositivity then Boron, but less then Be. [Note :- But as far as you must know Alkali Metals are most electropositive Elements ].
Q. Which has more metallic character beryllium or Aluminium?
Your friends are correct. Be is more metallic than Al. The standard reduction potential for Be is -1.85 V while that of aluminium is -1.66 V.
Q. Which group has more metallic character?
Metallic character increases down the group. As the atomic radius increases, the loss of electrons becomes easier. So the element at the bottom of group IA is most metallic.