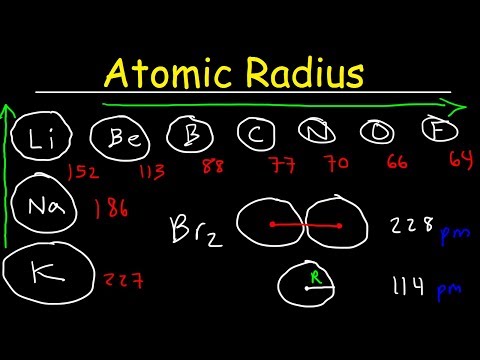
Helium has the smallest atomic radius. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.
Q. Which is larger AR or Kr?
Looking at the atomic radius trend of the periodic table, Kr has a larger radius than Ar.
Table of Contents
Q. Which noble gas has the largest radius?
As you go down a group, the atomic radius increases because you are adding energy levels that are farther away from the nucleus. Smallest to Largest: Helium….Largest to Smallest:
- Helium (highest ionization level in the periodic table)
- Neon.
- Argon.
- Krypton.
- Xenon.
- Radon.
Q. Why is there no atomic radii for noble gases?
Noble gases have an inert configuration, so they undergo repelsions, thus noble gases have a higher radius than 17th group elements. They do not have the highest radius in a period. Moreover, since noble gases are non reacting, so there exists a vanderwall radius between them.
Q. What atom has the largest atomic radius in Group 18?
radon
Q. What is the largest element in Group 18?
Oganesson has the highest atomic number and highest atomic mass of all known elements….
| Oganesson | |
|---|---|
| Atomic number (Z) | 118 |
| Group | group 18 (noble gases) |
| Period | period 7 |
| Block | p-block |
Q. Which is the smallest mass?
electron
Q. What is red quark?
Quarks have a color charge of red, green or blue and antiquarks have a color charge of antired, antigreen or antiblue. Gluons have a combination of two color charges (one of red, green, or blue and one of antired, antigreen, or antiblue) in a superposition of states which are given by the Gell-Mann matrices.
Q. Do quarks decay?
Up and down quarks can decay into each other by emission of a W boson (this is the origin of beta decay due to the fact that the W can, depending on its type, decay into electrons, positrons and electron (anti-)neutrinos, ). The current understanding of quarks is, that they are a fundamental particle.