Which example involves a phase change in which heat energy is released by the substance? freezing ice cream cooking a pot of soup melting ice under sunlight watching frost disappear into air.
Q. What is the relationship between evaporation and temperature?
Evaporation rates are higher at higher temperatures because as temperature increases, the amount of energy necessary for evaporation decreases. In sunny, warm weather the loss of water by evaporation is greater than in cloudy and cool weather.
Table of Contents
- Q. What is the relationship between evaporation and temperature?
- Q. Which best explains the relationship between heat energy and temperature *?
- Q. Which best describes the energy change that takes place during deposition quizlet?
- Q. Is initially present in a state where its molecules are far apart during a change of state its molecules slow down which change of state has most likely taken place quizlet?
- Q. What change in phase happens when ice cubes are heated?
- Q. Which phase changes requires an increase in energy?
- Q. During which phase change does water absorb the most heat?
- Q. When water is heated without rise of temperature it consumes?
- Q. What is the effect on boiling point of water when pressure is increased?
- Q. Why does water rise as its heated?
- Q. Why does the temperature of the water continue to rise after the flame is removed?
- Q. Can the change in temperature be negative?
- Q. How is heat related to changes in temperature?
- Q. What will happen to the water when it gets farther from the flame?
- Q. Can water make fire worse?
- Q. What happens to water when cooled?
- Q. Why is hot water used to extinguish fire?
- Q. Why does water kill fire?
- Q. Is water stronger than fire?
- Q. How do you kill a fire?
- Q. Is human skin flammable?
- Q. At what temperature does human skin catch fire?
- Q. Can a human body catch on fire?
Q. Which best explains the relationship between heat energy and temperature *?
Which best explains the relationship between heat energy and temperature? As heat energy increases and temperature remains constant, melting occurs. Heating a substance increases its kinetic energy, but melting and vaporization increase its potential energy.
Q. Which best describes the energy change that takes place during deposition quizlet?
Heat energy is absorbed by the substance. Which best describes the energy change that takes place during deposition? Heat energy is released by the substance. It is true because heat is released when a gas changes to a liquid.
Q. Is initially present in a state where its molecules are far apart during a change of state its molecules slow down which change of state has most likely taken place quizlet?
Water is initially present in a state where its molecules are far apart. During a change of state, its molecules slow down.
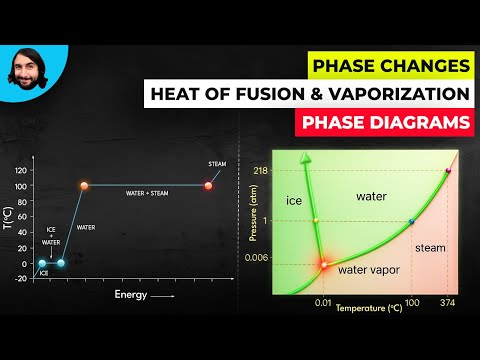
Q. What change in phase happens when ice cubes are heated?
The ice cubes are at the melting temperature of 0ºC. Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: first the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises.
Q. Which phase changes requires an increase in energy?
Evaporation involves a liquid becoming a gas and sublimation is the change of a solid directly to a gas. Phase changes require either the addition of heat energy (melting, evaporation, and sublimation) or subtraction of heat energy (condensation and freezing).
Q. During which phase change does water absorb the most heat?
If we are considering melting and boiling, the clear choice is boiling, which requires almost 7 times more energy than melting.
Q. When water is heated without rise of temperature it consumes?
When water is heated, latent heat of vaporisation is consumed to overcome the intermolecular forces of attraction at a constant temperature.
Q. What is the effect on boiling point of water when pressure is increased?
Atmospheric pressure influences the boiling point of water. When atmospheric pressure increases, the boiling point becomes higher, and when atmospheric pressure decreases (as it does when elevation increases), the boiling point becomes lower.
Q. Why does water rise as its heated?
But one big contributor to sea level rise is increasing global temperatures, which heat seas and cause something called thermal expansion of water. Thermal expansion happens when water gets warmer, which causes the volume of the water to increase.
Q. Why does the temperature of the water continue to rise after the flame is removed?
Temperature will be increases up to certain limit because of water molecules consumption heat energy and after flame out all molecules of water scattered at high velocity on the surface of water after some time because of room temperature temperature of water surface decrease and its gets cold out of certain limits…
Q. Can the change in temperature be negative?
An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. This heat is released into the surroundings, resulting in an overall negative quantity for the heat of reaction (qrxn<0).
Q. How is heat related to changes in temperature?
One of the major effects of heat transfer is temperature change: heating increases the temperature while cooling decreases it. Owing to the fact that the transferred heat is equal to the change in the internal energy, the heat is proportional to the mass of the substance and the temperature change.
Q. What will happen to the water when it gets farther from the flame?
Explanation: When heat is removed from water then there will be decrease in the kinetic energy of water particles. This is because on removing the heat, collisions between the particles will decrease as a result the energy will decrease. Therefore, kinetic energy of the water particles decreases.
Q. Can water make fire worse?
3. Do NOT pour water on the fire! Since oil and water do not mix, pouring water can cause the oil to splash and spread the fire even worse. In fact, the vaporizing water can also carry grease particles in it, which can also spread the fire.
Q. What happens to water when cooled?
When water is cooled, the water molecules move slower and get closer together. This makes cold water more dense than room temperature water. Since cold water is more dense, it sinks in the room temperature water. When water is heated, the water molecules move faster and spread out more.
Q. Why is hot water used to extinguish fire?
The process of fire extinguishing involves absorption of heat. Absorption of heat in converting hot water to steam is more than heat absorbed in heating cold water to the boiling temperature. Hence, hot water extinguishes fire more quickly than cold water.
Q. Why does water kill fire?
Why Does Water Extinguish Fire? When water extinguishes a fire, it does so because it doesn’t break down into its constituent elements. The water molecule doesn’t become hot enough to separate into hydrogen and oxygen and instead, has two potential actions on the fire that can put it out.
Q. Is water stronger than fire?
Water can be more powerful in its devastation than fire or wind, because it doesn’t always come by the bucketful. Sometimes it comes drop by drop. A single drop, repeated over and over can Wear away rock.
Q. How do you kill a fire?
firefighters need to have enough water and water pressure to fight a potential fire. Water is the best weapon to kill a fire.
Q. Is human skin flammable?
Skin is difficult to ignite, and usually chars in the presence of heat. It takes up to 10 minutes of direct flame for skin to crack open and start leaking out rendered fat. That’s because animal fat is a lot like candle wax: It needs a wick to burn. (Fat and wax happen to have nearly identical heats of combustion.
Q. At what temperature does human skin catch fire?
At 118 degrees, human skin can sustain first-degree burns; a second-degree burn injury can occur at a temperature of 131 degrees. Human skin is destroyed when temperatures reach 162 degrees.
Q. Can a human body catch on fire?
The human body isn’t especially flammable, she reasons, and has high water content. Surely the fire would be doused rather quickly even if the body did manage to catch fire. That’s why it takes flames of around 1600 degrees Fahrenheit over two hours or more to cremate human remains.