Two atoms with the same atomic number, but different mass numbers (same number of protons, different number of neutrons), are called isotopes, or isotopic nuclides. Having different numbers of neutrons changes the mass of these atoms, so isotopes have slight variations in their physical and chemical behavior.
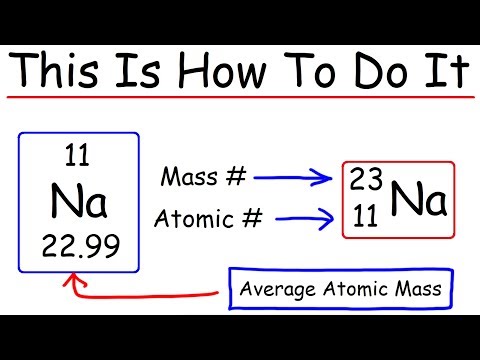
Q. How do you find the number of neutrons from atomic mass and atomic number?
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Table of Contents
- Q. How do you find the number of neutrons from atomic mass and atomic number?
- Q. How can we use atomic mass and atomic number to find the number of protons neutrons and electrons in an atom?
- Q. What is the atomic mass of neutrons?
- Q. What is the number of neutrons called?
- Q. What is the atomic mass of magnesium?
- Q. What is the main use of magnesium?
- Q. What is Oxygen’s weakness?
- Q. What is the formula of oxygen?
- Q. How do we know oxygen exists?
- Q. Where is oxygen most commonly found?
- Q. Is oxygen matter Yes or no?
- Q. Which country is the largest producer of oxygen in the world?
- Q. What’s the difference between medical air and oxygen?
- Q. Can you breathe in pure oxygen?
- Q. What’s the difference between oxygen and ventilator?
- Q. What is the difference between regular oxygen and pure oxygen?
Q. How can we use atomic mass and atomic number to find the number of protons neutrons and electrons in an atom?
The mass number = Number of neutrons+Z . Note that Z defines the identity of the element.
Q. What is the atomic mass of neutrons?
Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics.
Q. What is the number of neutrons called?
Atomic number and mass number The total number of protons and neutrons in an atom is called its mass number.
Q. What is the atomic mass of magnesium?
24.305 u
Q. What is the main use of magnesium?
Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. It is also added to molten iron and steel to remove sulfur. As magnesium ignites easily in air and burns with a bright light, it’s used in flares, fireworks and sparklers.
Q. What is Oxygen’s weakness?
Her weaknesses are when she is high up in the air and there is little oxygen in the air, it makes her dizzy.
Q. What is the formula of oxygen?
O2
Q. How do we know oxygen exists?
An English chemist, Joseph Priestley, independently discovered oxygen in 1774 by the thermal decomposition of mercuric oxide and published his findings the same year, three years before Scheele published.
Q. Where is oxygen most commonly found?
the atmosphere
Q. Is oxygen matter Yes or no?
Air is an example of the type of matter known as gas. Other common forms of matter are solids and liquids. If you analyze air, it consists mostly of nitrogen and oxygen, with smaller amounts of several other gases, including argon, carbon dioxide, and neon.
Q. Which country is the largest producer of oxygen in the world?
India
Q. What’s the difference between medical air and oxygen?
Medical Air – used in the ICU and NICU areas, medical air is supplied by a specific air compressor to patient care areas. Oxygen – a medical gas required in every healthcare setting, and is used for resuscitation and inhalation therapy.
Q. Can you breathe in pure oxygen?
Oxygen radicals harm the fats, protein and DNA in your body. This damages your eyes so you can’t see properly, and your lungs, so you can’t breathe normally. So breathing pure oxygen is quite dangerous.
Q. What’s the difference between oxygen and ventilator?
The patient is still responsible for his/her own breathing. Unlike the ventilator, oxygen therapy will not aid in respiration. Oxygenation also refers to the treatment of a patient by combining medication and other substances with oxygen. The treatment is a non-invasive measure to aid breathing.
Q. What is the difference between regular oxygen and pure oxygen?
Regular air is made up of 78% nitrogen, 21% oxygen, and 1% other gases. Oxygen concentrators are usually capable of delivering 90 to 95 percent pure oxygen. They deliver air in a continuous or intermittent flow.