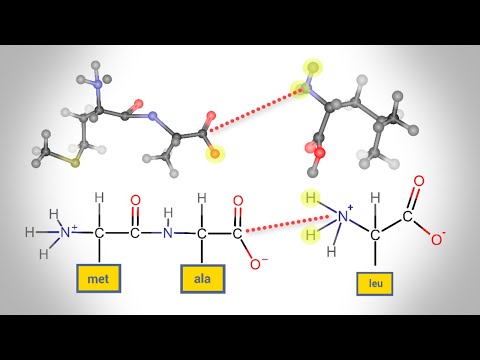
Amino acid molecules have two functional groups – the amine group (-NH 2) and a carboxyl group (-COOH). Proteins are formed in a condensation reaction when amino acid molecules join together and a water molecule is removed.
Q. When two amino acids are linked together a peptide bond is formed between the?
Peptide bonds form between the carboxyl group of one amino acid and the amino group of another through dehydration synthesis. A chain of amino acids is a polypeptide.
Table of Contents
- Q. When two amino acids are linked together a peptide bond is formed between the?
- Q. When amino acids are joined together by peptide bonds What is the result?
- Q. When two amino acids are linked together a peptide bond is formed between the quizlet?
- Q. What is it called when two amino acids are linked together?
- Q. What do two amino acids form?
- Q. What type of bond is being formed during translation to link two amino acids together?
- Q. What types of bond occurs between the amino acids quizlet?
- Q. What two types of bonds can form between cysteine amino acids?
- Q. What groups on the amino acids are always involved in hydrogen bonds?
- Q. What type of bond is found between two groups?
- Q. Which type of bond is the strongest?
- Q. What are the 4 types of bonds?
- Q. Which bonds are the strongest and weakest?
- Q. What is the weakest type of bond?
- Q. What is the weakest bond single double triple?
- Q. Which bonds are hardest to break?
- Q. How do you know when to use double or triple bonds?
- Q. Is a single or double bond stronger?
- Q. Is single bond the longest?
- Q. Why are single bonds stronger?
- Q. Is a shorter bond always stronger?
- Q. Why is a shorter bond stronger?
- Q. Is CH or C Br bond stronger?
- Q. What is the bond length of FF?
- Q. Which bond is longer FF or II?
- Q. What is the bond length of H Br?
- Q. Are double or triple bonds stronger?
- Q. Why is C2H2 a triple bond?
Q. When amino acids are joined together by peptide bonds What is the result?
Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Peptide bonds are formed by a biochemical reaction that extracts a water molecule as it joins the amino group of one amino acid to the carboxyl group of a neighboring amino acid.
Q. When two amino acids are linked together a peptide bond is formed between the quizlet?
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O). This is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids.
Q. What is it called when two amino acids are linked together?
the four atoms, nitrogen, hydrogen, carbon and oxygen that link the two amino acids together is called a peptide bond. two amino acids linked together in his way is called a dipeptide and a long chain of amino acids is called a polypeptide.
Q. What do two amino acids form?
A peptides is a molecule composed of two or more amino acids. The bond that holds together the two amino acids is a peptide bond, or a covalent chemical bond between two compounds (in this case, two amino acids).
Q. What type of bond is being formed during translation to link two amino acids together?
peptide bond
Q. What types of bond occurs between the amino acids quizlet?
A peptide bond is formed between two amino acids when the carboxyl group of one amino acids reacts with the amino group of the other amino acid, releasing a molecule of water. This is the dehydration reaction and usually occurs between amino acids.
Q. What two types of bonds can form between cysteine amino acids?
Ionic bonds form between two oppositely charged R groups, hydrogen bonds occur between two polar R groups, disulfide bonds form between two cysteine amino acids and hydrophobic interactions occur between two non-polar amino acids.
Q. What groups on the amino acids are always involved in hydrogen bonds?
Carboxyl and amino are the groups on the amino acids that are always involved in these bonds 12.
Q. What type of bond is found between two groups?
covalent bondA type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
Q. Which type of bond is the strongest?
Covalent Bonds
Q. What are the 4 types of bonds?
There are four types of chemical bonds essential for life to exist: Ionic Bonds, Covalent Bonds, Hydrogen Bonds, and van der Waals interactions.
Q. Which bonds are the strongest and weakest?
Of the 4 different types of chemical bonds, covalent bonds are known to be the strongest and the bonds formed via Van der Waals forces are known to be the weakest. The ranking is: Covalent bond > ionic bond > hydrogen bond > Van der Waals forces.
Q. What is the weakest type of bond?
ionic bond
Q. What is the weakest bond single double triple?
Triple bond has 50% s character (most) and hence has the least bond length and the most electronegativity! Hence,Triple Bond also has the most bond energy. So TRIPLE BOND IS THE STRONGEST BOND AND SINGLE BOND IS THE WEAKEST WHERE DOUBLE BOND LIES SOMEWHERE BETWEEN THE STRENGTHS OF SINGLE AND TRIPLE BOND.
Q. Which bonds are hardest to break?
Intramolecular covalent bonds, being around 98 percent stronger than intermolecular bonds, are the hardest to break and are very stable. It should be clear that since molecules exist, covalent bonds are stable. However when enough energy is provided to a molecule, the bonds may be broken.
Q. How do you know when to use double or triple bonds?
Re: When to use Double and Triple Bonds If a double or triple bond can be placed to lessen the number of different formal charges (for example, if the formal charge on an element is +1, and a double bond will change it’s formal charge to 0), then it should be added.
Q. Is a single or double bond stronger?
Double bonds are stronger than single bonds and they are characterized by the sharing of four or six electrons between atoms, respectively. Double bonds are comprised of sigma bonds between hybridized orbitals, and pi bonds between unhybridized p orbitals.
Q. Is single bond the longest?
Single bonds are the longest of the three types of covalent bonds as interatomic attraction is greater in the two other types, double and triple. The increase in component bonds is the reason for this attraction increase as more electrons are shared between the bonded atoms (Moore, Stanitski, and Jurs 343).
Q. Why are single bonds stronger?
bonds are weaker than sigma bonds since there is less overlap. Thus, two single bonds are stronger than a double bond, and more energy is needed to break two single bonds than a single double bond. The electron density lies above and below the atoms in a π bond.
Q. Is a shorter bond always stronger?
Yes, in general, a shorter bond length means a stronger bond. Atoms that are closer together are bonded more strongly to each other, and those that are far apart have a weak bond.
Q. Why is a shorter bond stronger?
A shorter bond length implies a stronger bond in general. Atoms that are closer together are more closely bound to each other and there is a weak bond between those that are further apart. If the number of electron pairs in the bond improves, the strength of a bond between two atoms increases.
Q. Is CH or C Br bond stronger?
Thus an H–F bond is stronger than an H–I bond, H–C is stronger than H–Si, H–N is stronger than H–P, H–O is stronger than H–S, and so forth….The Relationship between Bond Order and Bond Energy.
| Bond | (kJ/mol) |
|---|---|
| C-Br | 276 |
| C-I | 240 |
| C-C | 348 |
| C-N | 293 |
Q. What is the bond length of FF?
Common Bond Energies (D
| Bond | D (kJ/mol) | r (pm) |
|---|---|---|
| F-F | 155 | 142 |
| Cl-Cl | 240 | 199 |
| Br-Br | 190 | 228 |
| I-I | 148 | 267 |
Q. Which bond is longer FF or II?
Table 4.4. 2 compares the lengths of single covalent bonds with those of double and triple bonds between the same atoms. Without exception, as the number of covalent bonds between two atoms increases, the bond length decreases….Bond Length.
| Bond | Length (pm = 10−12 m) |
|---|---|
| F-F | 142 |
| Cl-Cl | 199 |
| Br-Br | 228 |
| I-I | 267 |
Q. What is the bond length of H Br?
Values greater than 2.50 are in the 2.50 bin. Values less than 1.40 are in the 1.40 bin.
| Species | Name | Bond Length (Å) |
|---|---|---|
| HBr- | hydrogen bromide anion | 2.394 |
| HBr | hydrogen bromide | 1.432 |
| HBr+ | hydrogen bromide cation | 1.469 |
| H2Br+ | protonated hydrogen bromide | 1.458 |
Q. Are double or triple bonds stronger?
Triple bonds are stronger than double bonds due to the the presence of two [latex]/pi[/latex] bonds rather than one. Each carbon has two sp hybrid orbitals, and one of them overlaps with its corresponding one from the other carbon atom to form an sp-sp sigma bond.
Q. Why is C2H2 a triple bond?
C2H2 Hybridization The 1s orbital of the Hydrogen atom overlaps with the Carbon atom’s 2p orbital atom, making it an sp hybridization. There are two-half filled 2p orbitals for each Carbon atom. These two orbitals form two pi bonds that result in the formation of triple bonds between carbon atoms.