There is an obvious pattern to look for: Atomic number increases as you move right along a row. Atomic number increases as you move down a column.
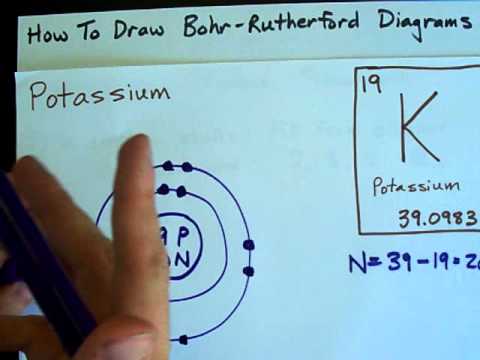
Q. What element is represented in this Bohr model?
Circulating round the nucleus are the electrons in various orbits of different energy levels. Electrons are negatively charged and represented by the symbol ‘e’. In the given image the number of protons are -6. Hence the element in question is Carbon as Carbon has the atomic number 6.
Table of Contents
- Q. What element is represented in this Bohr model?
- Q. Which atom is displayed by the Bohr diagram?
- Q. How does a Bohr diagram work?
- Q. Why is Bohr’s model wrong?
- Q. What are 3 features of a Bohr diagram?
- Q. What does Bohr’s theory say?
- Q. What are two limitations of the Bohr model for the atom?
- Q. What are advantages of Bohr’s model?
- Q. What are the limitations of Bohr Sommerfeld model?
- Q. What are the limitation of Rutherford’s model of atom?
- Q. What was Rutherford’s experiment called?
- Q. Who found the neutron?
- Q. Who discovered electrons are negative?
- Q. Who discovered positive and negative charge?
- Q. What is the definition of 1 Coulomb?
- Q. What are 3 examples of static?
Q. Which atom is displayed by the Bohr diagram?
The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (Figure 1). These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells.
Q. How does a Bohr diagram work?
In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy.
Q. Why is Bohr’s model wrong?
First, the Bohr model violates the Heisenberg Uncertainty Principle, since it states that electrons have a known radius and orbit. The Bohr Model also provides an incorrect value for the ground state orbital angular momentum and doesn’t work as well for creating diagrams of larger atoms.
Q. What are 3 features of a Bohr diagram?
Bohr’s model of the hydrogen atom is based on three postulates: (1) an electron moves around the nucleus in a circular orbit, (2) an electron’s angular momentum in the orbit is quantized, and (3) the change in an electron’s energy as it makes a quantum jump from one orbit to another is always accompanied by the …
Q. What does Bohr’s theory say?
The model states that electrons in atoms move in circular orbits around a central nucleus and can only orbit stably in certain fixed circular orbits at a discrete set of distances from the nucleus. These orbits are associated with definite energies and are also called energy shells or energy levels.
Q. What are two limitations of the Bohr model for the atom?
The Bohr Model is very limited in terms of size. Poor spectral predictions are obtained when larger atoms are in question. It cannot predict the relative intensities of spectral lines. It does not explain the Zeeman Effect, when the spectral line is split into several components in the presence of a magnetic field.
Q. What are advantages of Bohr’s model?
Advantages of Bohr’s Theory Thus, if electron is to be taken away from the nucleus, energy has to be supplied. The energy of the electron in n = 1 orbit is called the ground state energy; that in the n = 2 orbit is called the first excited state energy, etc.
Q. What are the limitations of Bohr Sommerfeld model?
(ii) It could not explain the distribution and arrangement of electrons in atoms. (iii) Sommerfeld’s model was unable to explain the spectra of alkali metals such as sodium, potassium etc. (iv) It could not explain Zeeman and Stark effect.
Q. What are the limitation of Rutherford’s model of atom?
Rutherford’s model was inadequate to explain the stability of an atom. It did not mention anything about the arrangement of an electron in orbit. As per Rutherford’s model, electrons revolve around the nucleus in a circular path.
Q. What was Rutherford’s experiment called?
Geiger–Marsden experiments
Q. Who found the neutron?
James Chadwick
Q. Who discovered electrons are negative?
Thomson’s
Q. Who discovered positive and negative charge?
Benjamin Franklin
Q. What is the definition of 1 Coulomb?
Coulomb, unit of electric charge in the metre-kilogram-second-ampere system, the basis of the SI system of physical units. It is abbreviated as C. The coulomb is defined as the quantity of electricity transported in one second by a current of one ampere.
Q. What are 3 examples of static?
Have you ever rubbed a balloon on your head and made your hair stand up? Have you ever walked across the carpet in your socks and received a shock from a doorknob? These are examples of static electricity.