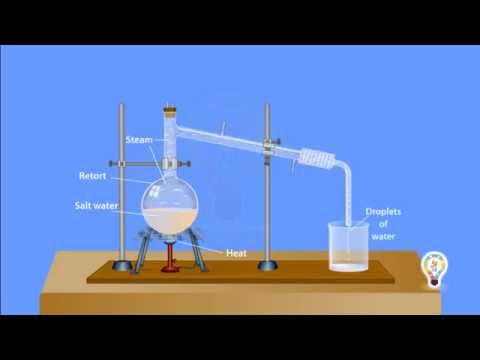
Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates.
Q. What is the best method to separate the components of ink?
By submerging chromatography paper in water, we can separate ink into its components. The water causes the ink molecules to travel up the paper. Depending on the mass of the molecule, various pigments will travel faster than others. This is the best method to separate the components of ink.
Table of Contents
- Q. What is the best method to separate the components of ink?
- Q. What method is used in identifying the dyes in an ink?
- Q. What marker works best chromatography?
- Q. Which method is used to obtain pure water ink?
- Q. How can we make water the purest liquid?
- Q. What is the purest form of liquid?
- Q. What are 3 pure liquids?
- Q. Is water the only pure liquid?
- Q. What are the 5 properties of liquid?
- Q. Which property shows that a liquid is pure?
- Q. How do you know if a sample is pure?
- Q. Do pure substances have higher boiling points?
- Q. Do pure substances have higher melting points?
- Q. Does melting point increase with pressure?
- Q. Which metal has the highest melting point?
- Q. Why do impure substances have lower melting points?
- Q. What increases melting point?
- Q. Why do impurities affect melting point?
- Q. What difference in melting point ranges is expected for impure and pure substances?
- Q. What is a pure substance melting point?
- Q. How do you determine melting point?
- Q. How does impurities affect boiling point?
- Q. What is effect of boiling point?
- Q. What factors affect boiling point?
- Q. How does impurities affect melting and boiling point?
- Q. What is effect of pressure on melting point?
- Q. How does the presence of impurities affect freezing and boiling point?
- Q. What two effects do impurities have on melting point?
Q. What method is used in identifying the dyes in an ink?
Ink Chromatography Lab Chromatography
Q. What marker works best chromatography?
The experiment works even better with filter paper or chromatography paper, but these are more expensive. Black pens are recommended because they usually have many different dyes in their ink. Students should be encouraged to try markers of different colors or food coloring (the green food coloring works well).
Q. Which method is used to obtain pure water ink?
Separate ink from water using a process called distillation. This is a process of separating two substances mixed together. Water vaporizes at a lower temperature than the ink pigment so if you heat them, the water evaporates, leaving the ink pigment in the flask.
Q. How can we make water the purest liquid?
Distilled water is produced by a process of distillation. Distillation involves boiling the water and then condensing the vapor into a clean container, leaving solid contaminants behind. Distillation produces very pure water.
Q. What is the purest form of liquid?
Distilled water is water that has been heated to the boiling point so that impurities are separated from the water, which itself becomes vapor or steam.
Q. What are 3 pure liquids?
Pure substances that are liquid under normal conditions include water, ethanol and many other organic solvents. Liquid water is of vital importance in chemistry and biology; it is believed to be a necessity for the existence of life. Inorganic liquids include water, magma, inorganic nonaqueous solvents and many acids.
Q. Is water the only pure liquid?
liquid state On Earth, water is the most abundant liquid, although much of the water with which organisms come into contact is not in pure form but is a mixture in which various substances are dissolved.
Q. What are the 5 properties of liquid?
Properties of Liquids
- Capillary Action.
- Cohesive and Adhesive Forces.
- Contact Angles.
- Surface Tension.
- Unusual Properties of Water.
- Vapor Pressure.
- Viscosity Viscosity is another type of bulk property defined as a liquid’s resistance to flow.
- Wetting Agents.
Q. Which property shows that a liquid is pure?
A pure liquid has a constant/fixed boiling point. With impurities, the boiling point of a substance is affected in two ways too: The boiling point is increased. The more impurities a substance contains, the higher its boiling point will be.
Q. How do you know if a sample is pure?
Impure substances tend to have a slightly lower melting point than the pure substance, and a broader melting temperature range. Pure substances can be identified by comparing the melting point found in the experiment with published reference data of what the melting point should be.
Q. Do pure substances have higher boiling points?
Because pure substances have constant properties throughout the whole sample it means that pure substances display a sharp melting point and a sharp boiling point.
Q. Do pure substances have higher melting points?
The melting point of a pure substance is always higher and has a smaller range than the melting point of an impure substance or, more generally, of mixtures.
Q. Does melting point increase with pressure?
Most liquids are less dense than the solid phase, so higher pressure increase the melting point. The dotted green line shows the melting point for water. Water is denser as a liquid, so higher pressures decrease the melting temperature.
Q. Which metal has the highest melting point?
tungsten
Q. Why do impure substances have lower melting points?
Foreign substances in a crystalline solid disrupt the repeating pattern of forces that holds the solid together. Therefore, a smaller amount of energy is required to melt the part of the solid surrounding the impurity. This explains the melting point depression (lowering) observed from impure solids.
Q. What increases melting point?
As heat is applied to a solid, its temperature will increase until the melting point is reached. More heat then will convert the solid into a liquid with no temperature change. When all the solid has melted, additional heat will raise the temperature of the liquid.
Q. Why do impurities affect melting point?
How impurities affect the melting point. If less energy is required, then this explains the melting point depression (lowering) observed from impure solids. The more impure the solid is, the more the structure is disrupted and the greater the variation in intermolecular forces in different areas of the solid.
Q. What difference in melting point ranges is expected for impure and pure substances?
A wide melting point range (more than 5°C) usually indicates that the substance is impure; a narrow melting point range (O. 5-2°C) usually indicates that the substance is fairly pure. If the two are identical, they should have the same melting point.
Q. What is a pure substance melting point?
Liquids. Pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid.
Q. How do you determine melting point?
Methods of Measuring Melting Point The most common and most basic method of determination is the capillary method. This method involves placing the sample in a capillary tube and running an experiment that will heat the sample until it reaches melting point. The melting point can then be recorded.
Q. How does impurities affect boiling point?
On adding an impurity, the vapor pressure of solution decreases. With an increase in concentration of solute, vapour pressure decreases, hence boiling point increases. This phenomenon is known as ‘elevation of boiling point’. For example – adding salt to water will lead to increase in its boiling point.
Q. What is effect of boiling point?
The boiling point increases with increased pressure up to the critical point, where the gas and liquid properties become identical. The boiling point cannot be increased beyond the critical point. Likewise, the boiling point decreases with decreasing pressure until the triple point is reached.
Q. What factors affect boiling point?
The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. When the atmospheric pressure is equal to the vapor pressure of the liquid, boiling will begin.
Q. How does impurities affect melting and boiling point?
The reason for impurities lowering the melting point yet increasing the boiling point is because the impurities stabilise the liquid phase, making it more energetically favourable. This extends the liquid range to lower temperatures (lowering the melting point) and to higher temperatures (raising the boiling point).
Q. What is effect of pressure on melting point?
As the pressure of substance increases, particles tends to remains compacted, increasing of pressure during melting hindering in melting process, makes it difficult to overcome the strong force of attraction, i.e. more thermal energy is required. That’s why the melting point increases as the pressure increase.
Q. How does the presence of impurities affect freezing and boiling point?
Presence of impurities in the substance affects the boiling point and freezing points of a substance. It makes variation in the freezing points and boiling points by making them low or high. Substances that have no impurities or Pure substances have proper boiling and freezing points .
Q. What two effects do impurities have on melting point?
Impurities present in a solid organic compound tend to have 2 effects on the melting point. First, they tend to lower the overall melting point of the compound versus the value for pure material. Second, they tend to increase the range of the melting point values.