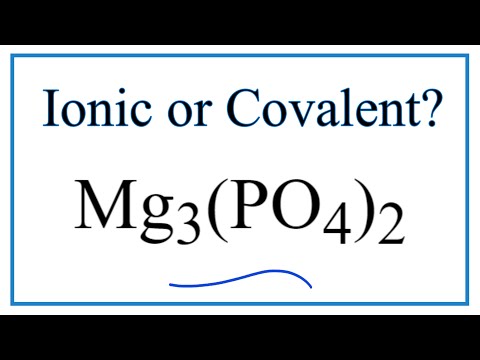Magnesium phosphate is Mg3(PO4)2. Okay, onto how I found that: Magnesium phosphate is an ionic compound, because firstly, there is no numerical prefix on the second word of the name, so we know it is not a covalent compound.
Q. What does 4 mean in the formula 4Na2SO3?
Answer: The ‘4’ in front of sodium sulfate formula indicates that there are four molecules of sodium sulfate.
Table of Contents
- Q. What does 4 mean in the formula 4Na2SO3?
- Q. What does the 2 mean in h2o?
- Q. What do the two mean in the formula 5 mg 3 PO 42?
- Q. Is ca3 PO4 2 soluble in water?
- Q. When Mg3 PO4 2 dissolves how many ions are there all together?
- Q. Is magnesium phosphate soluble or insoluble?
- Q. Is mg3po4 soluble or insoluble?
- Q. What kind of bond is magnesium phosphate?
- Q. What is the formula of magnesium phosphate?
- Q. What is the relationship between phosphorus and magnesium?
- Q. What is magnesium phosphate used for?
- Q. Can you overdose on magnesium phosphate?
- Q. Does magnesium phosphate make you sleep?
- Q. What are the side effects of magnesium?
Q. What does the 2 mean in h2o?
2. O is the chemical formula for water, meaning that each of its molecules contains one oxygen and two hydrogen atoms.
Q. What do the two mean in the formula 5 mg 3 PO 42?
The compound magnesium phosphate has the chemical formula Mg3(PO4)2. In this compound, phosphorous and oxygen act together as one charged particle, which is connected to magnesium, the other charged particle.
Q. Is ca3 PO4 2 soluble in water?
It is insoluble in ethanol, acetic acid but soluble in dilute nitric acid and hydrochloric acid. It slightly dissolves in water.
Q. When Mg3 PO4 2 dissolves how many ions are there all together?
Magnesium phosphate is Mg3(PO4)2. The subscripts are calculated using the LCM (least common multiple) of the ionic charges: Mg ions are +2 and PO4 ions are -3, so the LCM is 6. This means that 3 Mg ions are needed to combine with 2 PO4 ions.
Q. Is magnesium phosphate soluble or insoluble?
h) All phosphates are insoluble except those of sodium, potassium and ammonium. Some hydrogen phosphates, such as Ca(H2PO4)2, are soluble. I) All sulfides are insoluble except those of ammonium, sodium, calcium, potassium, magnesium, barium and strontium. All of these are slightly hydrolyzed in water.
Q. Is mg3po4 soluble or insoluble?
Is Mg3(PO4)2 ( Magnesium Phosphate ) Soluble or Insoluble in water ?
| Soluble List | |
|---|---|
| Mg(C2H3O2)2 | Insoluble |
| Mg(OH)2 ( Magnesium hydroxide ) | Insoluble |
| Mg3(PO4)2 ( Magnesium Phosphate ) | Insoluble |
| MgCO3 ( Magnesium carbonate ) | Insoluble |
Q. What kind of bond is magnesium phosphate?
Magnesium phosphate is an ionic compound composed of the magnesium cation(Mg2+) and phosphate anion(PO43-).
Q. What is the formula of magnesium phosphate?
Mg3(PO4)2
Q. What is the relationship between phosphorus and magnesium?
Magnesium and Phosphorus As calcium has an inverse relationship to phosphorus, magnesium also has an inverse relationship to phosphorus. The kidneys play an important role in magnesium reabsorption and excretion. As previously discussed, the kidneys also help regulate the phosphorus and calcium levels in the body.
Q. What is magnesium phosphate used for?
Magnesium phosphate is widely used in medication in order to support the relaxation of muscles. This compound is also used to prevent the cramping of muscles. Magnesium phosphate is also used to prevent vitamin E deficiency.
Q. Can you overdose on magnesium phosphate?
Magnesium is essential for well-being, but too much can cause problems, including digestive issues, lethargy, and an irregular heartbeat. In rare cases, a magnesium overdose can be fatal. Magnesium toxicity is rare in otherwise healthy people, and levels are more likely to be low than high.
Q. Does magnesium phosphate make you sleep?
It Can Help Your Body and Brain Relax In order to fall asleep and stay asleep, your body and brain need to relax. On a chemical level, magnesium aids this process by activating the parasympathetic nervous system, the system responsible for getting you calm and relaxed ( 6 ).
Q. What are the side effects of magnesium?
In some people, magnesium might cause stomach upset, nausea, vomiting, diarrhea, and other side effects. When taken in very large amounts (greater than 350 mg daily), magnesium is POSSIBLY UNSAFE.