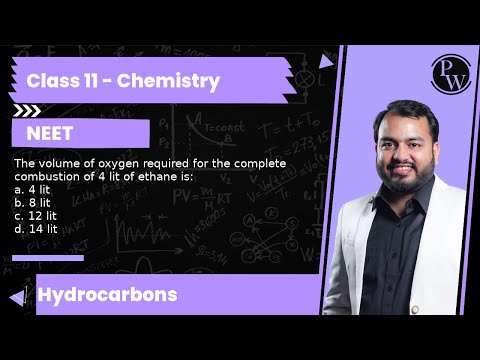
14 litres
Q. How many liters of air are needed to complete the combustion?
Answer. Answer: 1,495.11 L volume of air will be required for the complete combustion of octane vapors of 25 L.
Table of Contents
- Q. How many liters of air are needed to complete the combustion?
- Q. Which fuel requires the lowest amount of excess air for combustion?
- Q. What is the ideal air fuel ratio for efficient combustion?
- Q. How do you calculate excess air?
- Q. What is the excess air?
- Q. What is the advantage of using an excess of air?
- Q. What is the importance to have optimum excess air factor?
- Q. Why do gas furnace efficiencies jump from 80 percent to 90 percent?
- Q. How do I control the excess air in my boiler?
- Q. What is the purpose of excess air in furnace combustion quizlet?
- Q. What controls the amount of gas entering the burner quizlet?
- Q. How does excess air affect the vent gas CO2 percentage quizlet?
- Q. What type of flame is characteristic of incomplete combustion?
- Q. Does charcoal burn with a flame?
- Q. What is the difference between complete and incomplete combustion?
- Q. What temperature is a blue flame?
- Q. Is Blue Fire hotter?
Q. Which fuel requires the lowest amount of excess air for combustion?
Combustion efficiency Excess air depends on type of fuel. Normally solid fuels require more excess air than liquid fuels and gaseous fuels. Gaseous fuels require least amount of excess air.
Q. What is the ideal air fuel ratio for efficient combustion?
around 14.7:1
Q. How do you calculate excess air?
When the air/fuel ratio is optimized, the resulting energy savings usually ranges from 5% to >25%. For example, if the oxygen dry reading in flue gas is 2.5%, then the excess-air calculation would be: 0.895 x 0.025 / (0.21-0.025) = 12.1% excess air.
Q. What is the excess air?
There is a theoretical amount of fresh air that when mixed with a fixed amount of fuel, and burnt will result in perfect combustion. In this situation all of the fuel will have been properly burnt and all of the oxygen in the air will have been consumed.
Q. What is the advantage of using an excess of air?
Excess air increase the amount of oxygen to the combustion and the combustion of fuel. The combustion efficiency increases with increased excess air – until the heat loss in the excess air is larger than the heat provided by more efficient combustion.
Q. What is the importance to have optimum excess air factor?
Operating your boiler with an optimum amount of excess air will minimize heat loss up the stack and improve combustion efficiency. Combustion efficiency is a measure of how effectively the heat content of a fuel is transferred into usable heat.
Q. Why do gas furnace efficiencies jump from 80 percent to 90 percent?
Why do gas furnace efficiencies jump from 80% to 90%? Efficiencies over 80% cause condensation but the flue gas is still too hot for PVC vent pipe until the efficiency reaches 90%.
Q. How do I control the excess air in my boiler?
Maintaining low excess air levels at all firing rates ensures that boilers’ fuel usage and operating costs remain reasonable. A large percentage of industrial boilers use modulating burners with single-point positioning control consisting of a mechanically-linked fuel valve and a combustion air damper.
Q. What is the purpose of excess air in furnace combustion quizlet?
What is the purpose of excess air in furnace combustion? Excess air insures that all the gas is burned by making sure there is plenty of oxygen available.
Q. What controls the amount of gas entering the burner quizlet?
A precisely drilled hole that controls the amount of gas entering a burner is called: An orifice.
Q. How does excess air affect the vent gas CO2 percentage quizlet?
Excess air is added to insure complete combustion. This reduces the CO2 percentage by adding more air than is used in the combustion process.
Q. What type of flame is characteristic of incomplete combustion?
With complete combustion, methane burns with a blue flame color (natural gas blue flame) and burns at a temperature of around 1,960°C. Natural gas flame color orange indicates incomplete combustion.
Q. Does charcoal burn with a flame?
The substances which vapourise during burning, give flames. For example, kerosene oil and molten wax rise through the wick and are vapourised during burning and form flames. Charcoal, on the other hand, does not vapourise and so does not produce a flame.
Q. What is the difference between complete and incomplete combustion?
Complete combustion takes place in the presence of a sufficient amount of oxygen while an incomplete combustion reaction takes place when there is an insufficient amount of oxygen supply.
Q. What temperature is a blue flame?
Blue Flame Means Complete Combustion With complete combustion, an LPG (Propane) flame burns at a temperature of around 1,980°C. For Natural Gas (Methane), the temperature is about 1,960°C, according to the flame color temperature chart.
Q. Is Blue Fire hotter?
The color blue indicates a temperature even hotter than white. Blue flames have more oxygen and get hotter because gases burn hotter than organic materials, such as wood. When natural gas is ignited in a stove burner, the gases quickly burn at a very high temperature, yielding mainly blue flames.