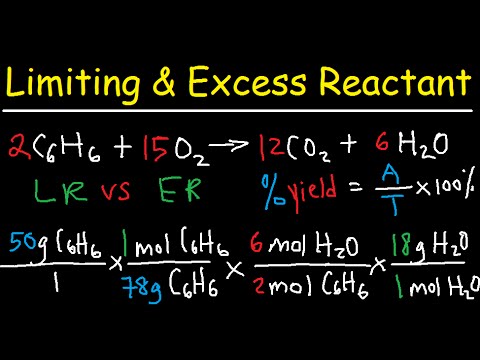The actual yield is the amount of product actually obtained in a chemical reaction. In conclusion, the theoretical yield of CO2 depends entirely on the limiting reagent, the quantity of product (CO2) that is obtained is through stoichiometric calculations of the reaction using the limiting reagent.
Q. What is the formula for yield?
Yield is calculated as: Yield = Net Realized Return / Principal Amount. For example, the gains and return on stock investments can come in two forms.
Table of Contents
- Q. What is the formula for yield?
- Q. Why is it important to use the limiting reactant to calculate the theoretical yield?
- Q. What is the ratio of the actual yield to the theoretical yield multiplied by 100 %?
- Q. Why is the actual yield of a reaction almost always smaller than the theoretical yield?
- Q. Which reactant hydrogen or oxygen is the limiting reactant in this case?
- Q. Is oxygen a limiting reactant?
- Q. Can both reactants be limiting?
- Q. What’s the limiting reactant in water?
- Q. What is the limiting reactant in CO2?
- Q. What is a limiting reactant in a reaction?
- Q. How do you solve excess reactant problems?
- Q. Why is excess reactant important?
- Q. Which is excess reactant?
- Q. How do excess reagent affects the chemical reaction?
- Q. What is the limiting reactant and theoretical yield?
- Q. What is limiting reagent explain with an example?
- Q. How do you know what is the limiting reagent?
- Q. What is the meaning of limiting reagent?
- Q. What is limiting and excess reagent?
- Q. Why are limiting and excess reactants important?
- Q. What is percentage yield and its importance in making a solution?
- Q. Why is product yield important?
- Q. What is the importance of percentage yield?
- Q. How do I calculate the yield of a reaction?
- Q. What can affect actual yield?
- Q. What does percent yield indicate?
- Q. What is mol ratio?
- Q. Is a high or low percent yield better?
- Q. Does percent yield indicate purity?
- Q. What is the difference between percent yield and percent error?
- Q. What is the actual value in percent error?
- Q. Is a high percent error good or bad?
- Q. What causes percent error?
Q. Why is it important to use the limiting reactant to calculate the theoretical yield?
The limiting reactant in a chemical reaction is the reactant that determines the amount of product that can be formed. The theoretical yield is the maximum amount of product that could be formed based on stoichiometry calculations.
Q. What is the ratio of the actual yield to the theoretical yield multiplied by 100 %?
Chemistry Chapter 9 Matching
| A | B |
|---|---|
| Mol B | Mass A –> Mol A –> _____ _ |
| Molar Mass | the mass in grams of one mole of a substance, relates the mass of a substance to the amount in moles of the substance. |
| Percent Yield | is the ratio of the actual yeild to the theoretical yeild multiplied by 100% |
Q. Why is the actual yield of a reaction almost always smaller than the theoretical yield?
Actual yield in a reaction is almost always less than the theoretical yield, primarily because losses of the substances involved may occur anywhere in an experiment. Otherwise, there can be so many possibilities that can be reasoned out depending on the reaction.
Q. Which reactant hydrogen or oxygen is the limiting reactant in this case?
In this example, hydrogen is the limiting reagent and oxygen is the excess reagent. The amount of product formed is limited by the amount of hydrogen. In a chemical reaction, reactants that are not used up when the reaction is finished are called excess reagents.
Q. Is oxygen a limiting reactant?
Oxygen (O2) produces less water (H2O) than hydrogen (H2) therefore oxygen is the limiting reactant.
Q. Can both reactants be limiting?
Two reactants cannot limit each other. There is too little of one or the other, or they are present in the correct ratio, where they both would be used up completely and neither is limiting the other.
Q. What’s the limiting reactant in water?
hydrogen
Q. What is the limiting reactant in CO2?
Because the calculated mole ratio exceeds the theoretical mole ratio, we have more NH3 than needed to completely consume the available CO2. The limiting reagent, therefore, is CO2 and NH3 is the excess reagent.
Q. What is a limiting reactant in a reaction?
The limiting reagent is the reactant that is used up completely. This stops the reaction and no further products are made. Given the balanced chemical equation that describes the reaction, there are several ways to identify the limiting reagent.
Q. How do you solve excess reactant problems?
Strategy
- Write the chemical equation.
- Calculate the moles of product from the first reactant.
- Calculate the moles of product from the second reactant.
- Identify the limiting reactant and the excess reactant.
- Calculate the mass of excess reactant used up.
- Calculate the mass of unused excess reactant.
Q. Why is excess reactant important?
A good way to ensure that one reactant fully reacts is to use an excess of the other reactant. The other reactant becomes a limiting factor and controls how much of each product is produced. While using excess reactants can help to increase percentage yields, this is at the expense of atom economy.
Q. Which is excess reactant?
An excess reactant is a reactant present in an amount in excess of that required to combine with all of the limiting reactant. It follows that an excess reactant is one remaining in the reaction mixture once all the limiting reactant is consumed.
Q. How do excess reagent affects the chemical reaction?
The reactant that acts as a limiting reagent will be consumed first by the reaction, in essence leaving the other reactant(s) in excess. This implies that the amounts of products the reaction forms will depend on the limiting reagent.
Q. What is the limiting reactant and theoretical yield?
A limiting reagent is a chemical reactant that limits the amount of product that is formed. The limiting reagent gives the smallest yield of product calculated from the reagents (reactants) available. This smallest yield of product is called the theoretical yield.
Q. What is limiting reagent explain with an example?
What is Limiting Reagents? The reactant that is entirely used up in a reaction is called as limiting reagent. In the reaction given above, 3 moles of Hydrogen gas are required to react with 1 mole of nitrogen gas to form 2 moles of ammonia.
Q. How do you know what is the limiting reagent?
The reactant that produces a lesser amount of product is the limiting reagent. The reactant that produces a larger amount of product is the excess reagent. To find the amount of remaining excess reactant, subtract the mass of excess reagent consumed from the total mass of excess reagent given.
Q. What is the meaning of limiting reagent?
The limiting reagent is the reactant that is used up completely. This stops the reaction and no further products are made. One way to determine the limiting reagent is to compare the mole ratios of the amounts of reactants used. This method is most useful when there are only two reactants.
Q. What is limiting and excess reagent?
The limiting reagent in a chemical reaction is the reactant that will be consumed completely. Therefor it limits the reaction from continuing. Excess Reagent. The excess reagent is the reactant that could keep reacting if the other had not been consumed.
Q. Why are limiting and excess reactants important?
The limiting reactant is very important since it stops the reaction…it controls the amount of product made. Identifying the Limiting Reactant and Theoretical Yield: Limiting reactant problems in our class will tell you how much of more than one reactant is used in the reaction.
Q. What is percentage yield and its importance in making a solution?
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage: Percent Yield=Actual YieldTheoretical Yield×100% Percent yield is very important in the manufacture of products. Much time and money is spent improving the percent yield for chemical production.
Q. Why is product yield important?
Importance. Percentage yield is important because: chemical reactions very often form by-products as well as the intended product. in most reactions, not all of the reactants actually react.
Q. What is the importance of percentage yield?
The percentage yield of a chemical reaction is an important consideration in industrial chemistry. It can be calculated to compare the yield (quantity) of product actually obtained with what could have been obtained in theory, if all of the reactants were converted with no loss or waste.
Q. How do I calculate the yield of a reaction?
Calculate the reaction yield. % yield = 100 x actual yield / theoretical yield = 100 x 1.90 / 2.61 = 72.8% ~72% (2 sf)
Q. What can affect actual yield?
In this case the mass of products formed (the actual yield) is less than the theoretical yield….
| Temperature: | Temperature often has a tremendous influence on a reaction. The speed (or rate) of a reaction usually increases with temperature |
|---|---|
| Pressure: | Gas pressure can also affect reactions. |
Q. What does percent yield indicate?
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. It’s possible for percent yield to be over 100%, which means more sample was recovered from a reaction than predicted.
Q. What is mol ratio?
A mole ratio is a conversion factor that relates the amounts in moles of any two substances in a chemical reaction. The numbers in a conversion factor come from the coefficients of the balanced chemical equation.
Q. Is a high or low percent yield better?
Percent yield compares the hands-on results to the calculated predictions. A higher percent yield might signal that your product is being contaminated by water, excess reactant, or another substances. A lower percent yield might signal that you mis-measured a reactant or spilled a portion of your product.
Q. Does percent yield indicate purity?
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage. However, percent yields greater than 100% are possible if the measured product of the reaction contains impurities that cause its mass to be greater than it actually would be if the product was pure.
Q. What is the difference between percent yield and percent error?
The actual yield of a reaction is the actual amount of product that is produced in the laboratory. The percentage of the theoretical yield that is actually produced (actual yield) is known as the percent yield. Percent error is always an absolute value… no negatives!
Q. What is the actual value in percent error?
Key Points: Percent Error The purpose of a percent error calculation is to gauge how close a measured value is to a true value. Percent error (percentage error) is the difference between an experimental and theoretical value, divided by the theoretical value, multiplied by 100 to give a percent.
Q. Is a high percent error good or bad?
Percent errors tells you how big your errors are when you measure something in an experiment. Smaller percent errors mean that you are close to the accepted or real value. For example, a 1% error means that you got very close to the accepted value, while 45% means that you were quite a long way off from the true value.
Q. What causes percent error?
Common sources of error include instrumental, environmental, procedural, and human. All of these errors can be either random or systematic depending on how they affect the results. Instrumental error happens when the instruments being used are inaccurate, such as a balance that does not work (SF Fig. 1.4).