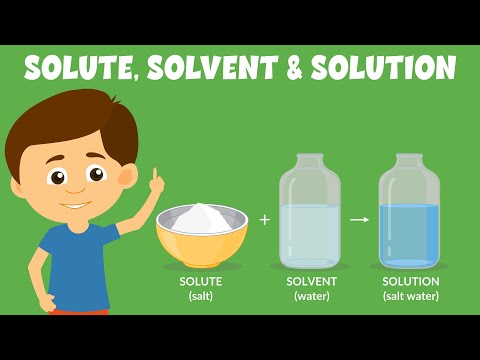
solvent: the substance in which a solute dissolves to produce a homogeneous mixture. solute: the substance that dissolves in a solvent to produce a homogeneous mixture.
Q. What is insoluble or soluble solution?
A solution is made up of a solvent (such as water) in which one or more solutes have been dissolved. In a solution, the solute looks as if it disappears into the solvent. Not all substances dissolve in water. Those that dissolve are called soluble substances; those that do not dissolve are called insoluble substances.
Table of Contents
- Q. What is insoluble or soluble solution?
- Q. What is soluble solvent and solute?
- Q. What are insoluble solvents?
- Q. What is soluble and insoluble in water?
- Q. How do you know if a solution is soluble or insoluble?
- Q. What is a water soluble liquid?
- Q. What is the difference between soluble and insoluble?
- Q. What are examples of insoluble substances?
- Q. Which foods have insoluble fiber?
- Q. Which salt is insoluble?
Q. What is soluble solvent and solute?
Solubility is a property referring to the ability for a given substance, the solute, to dissolve in a solvent. The solvent is often a solid, which can be a pure substance or a mixture. The species that dissolves, the solute, can be a gas, another liquid, or a solid.
Q. What are insoluble solvents?
Non-polar solvent insoluble in water are Pet-Ether, dietyl ether, n-hexane, benzene ,toluene, xylene, ethyl acetate etc.
Q. What is soluble and insoluble in water?
Substances that dissolve in water are called soluble substances. When you mix sugar with water, the sugar dissolves to make a transparent solution. Substances that do not dissolve in water are called insoluble substances. When you mix sand or flour with water, they do not dissolve.
Q. How do you know if a solution is soluble or insoluble?
Solubility Rules
- Salts containing Group I elements (Li+, Na+, K+, Cs+, Rb+) are soluble .
- Salts containing nitrate ion (NO3-) are generally soluble.
- Salts containing Cl -, Br -, or I – are generally soluble.
- Most silver salts are insoluble.
- Most sulfate salts are soluble.
- Most hydroxide salts are only slightly soluble.
Q. What is a water soluble liquid?
Soluble liquids are water soluble formulations of active ingredients in water or in polar solvents. In many cases actives are solutions of alkaline salts, thus sequestering agents are necessary to avoid flocculation of salts in field-rate dilution water.
Q. What is the difference between soluble and insoluble?
There are two types namely: soluble and insoluble fibers. The primary and perhaps the most obvious difference between them is that soluble fibers are literally soluble (can be dissolved) in liquid or water while insoluble fibers aren’t. The two are digested differently.
Q. What are examples of insoluble substances?
Examples of compounds that are considered insoluble in water are the: Carbonates (except group I, ammonium, and uranyl compounds) Sulfites (except group I and ammonium compounds) Phosphates (except for some group 1 and ammonium compounds; lithium phosphate is soluble) Hydroxides (multiple exceptions) Oxides (multiple exceptions) Sulfides (except group I, group II, and ammonium compounds)
Q. Which foods have insoluble fiber?
Soluble fiber is found in oat bran, barley, nuts, seeds, beans, lentils, peas, and some fruits and vegetables. It is also found in psyllium, a common fiber supplement. Some types of soluble fiber may help lower risk of heart disease. Insoluble fiber is found in foods such as wheat bran, vegetables, and whole grains.
Q. Which salt is insoluble?
Most chloride salts are readily soluble in water, but mercurous chloride (calomel) and silver chloride are insoluble, and lead chloride is only slightly soluble. Some chlorides, e.g., antimony chloride and bismuth chloride , decompose in water, forming oxychlorides.