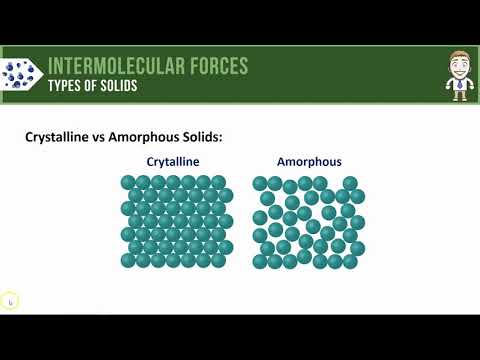
Solids can be classified into two types: crystalline and amorphous. Crystalline solids are the most common type of solid. They are characterized by a regular crystalline organization of atoms that confer a long-range order. Amorphous, or non-crystalline, solids lack this long-range order.
Q. What are the 3 properties of solid?
Explanation:
Table of Contents
- Q. What are the 3 properties of solid?
- Q. What are the 5 properties of solid?
- Q. Which type of solid is strongest?
- Q. What are 4 types of solids?
- Q. Which type of solid IMF is the strongest?
- Q. Which of the following interactions is the strongest?
- Q. What is the strongest intermolecular force present in carbon monoxide?
- Q. What is the major attractive force in ch4?
- Q. Is f2 London dispersion?
- Q. What is the strongest molecular interaction?
- Q. Which of the following has the strongest van der Waals interactions?
- Q. Which of the following are the strongest molecular interactions quizlet?
- Q. Why are ionic bonds weak in water?
- Q. When two atoms are equally electronegative they will interact to form?
- A solid has a definite shape and volume.
- Solids in general have higher density.
- In solids, intermolecular forces are strong.
- Diffusion of a solid into another solid is extremely slow.
- Solids have high melting points.
Q. What are the 5 properties of solid?
Solids have many different properties, including conductivity, malleability, density, hardness, and optical transmission, to name a few.
Q. Which type of solid is strongest?
Covalent bonds are very strong, so covalent network solids typically have the highest melting points out of all four types of solids. They usually don’t conduct electricity because valence electrons are localized within covalent bonds.
Q. What are 4 types of solids?
There are four types of crystalline solids: ionic solids, molecular solids, network covalent solids and metallic solids.
Q. Which type of solid IMF is the strongest?
hydrogen bonding
Q. Which of the following interactions is the strongest?
ionic interactions
Q. What is the strongest intermolecular force present in carbon monoxide?
The dipole-dipole attractions between CO molecules are comparably stronger than the dispersion forces between nonpolar N2 molecules, so CO is expected to have the higher boiling point.
Q. What is the major attractive force in ch4?
The only intermolecular forces in methane are London dispersion forces. The major intermolecular forces would be dipole-dipole forces and London dispersion forces. The electronegativities of C and H are so close that C-H bonds are nonpolar. There are no bond dipoles and no dipole-dipole interactions.
Q. Is f2 London dispersion?
The London Dispersion Forces in I2 are strong enough to keep I2 solid at room temperature; where as, F2 is a gas at room temperature. In general London Dispersion Forces are considered to be the weakest intermolecular force; however, London Dispersion Forces become very important for larger molecules.
Q. What is the strongest molecular interaction?
Dipole-dipole interactions are the strongest intermolecular force of attraction.
Q. Which of the following has the strongest van der Waals interactions?
The van der Waals forces are stronger in hexane than in pentane because hexane has a larger surface area to interact with neighboring molecules. The stronger intermolecular attraction holds molecules together more tightly, decreasing the vapor pressure of hexane and giving it a higher boiling point than pentane.
Q. Which of the following are the strongest molecular interactions quizlet?
Terms in this set (6)
- Ionic Bonds. – is the strongest bonds – when one atom takes the electrons of another atoms.
- Covalent Bonds. when there is a charging of the electrons between two atoms.
- Van der Waals.
- London Dispersion Force.
- Dipole-Dipole interactions.
- Hydrogen Bonding.
Q. Why are ionic bonds weak in water?
Ionic bonds are stronger than covalent bonds, but when dissolved in water, they become much weaker because ions separate and are surrounded by water molecules.
Q. When two atoms are equally electronegative they will interact to form?
Question: When Two Atoms Are Equally Electronegative, They Will Interact To Form Ionic Bonds Equal Numbers Of Isotopes. Nonpolar Covalent Bonds. Polar Covalent Bonds. Oions Atoms W, X, Y & Z Have Atomic Numbers Of 9, 10, 11 & 12 Respectively.