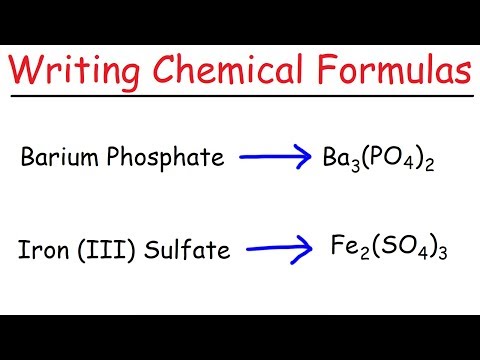
Compounds are represented by chemical formulas. Elements in a compound are represented by chemical symbols, and the ratio of different elements is represented by subscripts. There are different rules for writing the chemical formulas for ionic and covalent compounds.
Q. WHAT IS elements and compounds with example?
Elements, mixtures and compounds Common examples include carbon, sulfur, oxygen, iron, copper, aluminium. Elements are represented by symbols. Compounds are substances made from atoms of different elements joined by chemical bonds. They can only be separated by a chemical reaction.
Table of Contents
- Q. WHAT IS elements and compounds with example?
- Q. What are the types of compounds?
- Q. What is a compound class 9?
- Q. What are the properties of a compound class 9?
- Q. What are elements and compounds Class 9?
- Q. What are bio unlimited elements?
- Q. What are scavenged elements?
- Q. What are three examples of trace elements found in the ocean?
Q. What are the types of compounds?
Types of Compounds
- Metal + Nonmetal —> ionic compound (usually)
- Metal + Polyatomic ion —> ionic compound (usually)
- Nonmetal + Nonmetal —> covalent compound (usually)
- Hydrogen + Nonmetal —> covalent compound (usually)
Q. What is a compound class 9?
Compound is a pure substance made up of two or more elements combined chemically in a definite ratio. Characteristics: Constituent elements can be separated by chemical process.
Q. What are the properties of a compound class 9?
- Components in a compound are present in a definite proportion.
- It has a homogeneous composition.
- Particles in a compound are of one kind.
- A compound is made up of one or more atoms of the same or different elements.
- In a compound the elements are present in a fixed ratio by mass.
Q. What are elements and compounds Class 9?
Elements are pure substances which are composed of only one type of atom. Compound are substances which are formed by two or more different types of elements that are united chemically in fixed proportions.
Q. What are bio unlimited elements?
biolimiting elements A few elements (N, P, and Si) which are almost totally depleted in surface waters, relative to deep water, by biological activity. As these elements are essential to living organisms, the depletion limits further biological production until the scarce elements are replaced, e.g. by upwelling.
Q. What are scavenged elements?
Scavenged Elements are those that react with other particles and are adsorbed to the particle surface. When the particles sink, those elements are removed to the sediment .
Q. What are three examples of trace elements found in the ocean?
These three elements—Fe, Hg and Ra—epitomize the importance of trace elements in the oceans as nutrients, contaminants and tracers.