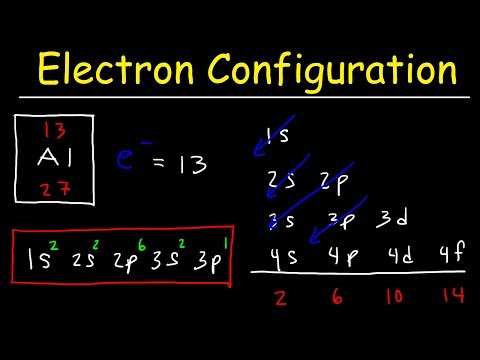
All you can say is that if an electron is in a particular orbital it will have a particular definable energy. Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The “1” represents the fact that the orbital is in the energy level closest to the nucleus.
Q. Is 1s possible?
Re: Confusion about why there is no 1p, 1d, or 1f orbital? In the first shell, there is only the 1s orbital, the shell can have a maximum of only 2 electrons. Therefore, the 1p, 1d, or 1f does not exist. The quantum number “n” must be larger than angular momentum quantum number.
Table of Contents
- Q. Is 1s possible?
- Q. Is 2s possible?
- Q. Why is it called Hund’s rule?
- Q. What is maximum multiplicity?
- Q. Is called Rule of maximum multiplicity?
- Q. Why Hund’s rule is called maximum multiplicity rule?
- Q. What is the spin multiplicity of oxygen?
- Q. What is Auf Bau principle what is Hund’s rule?
- Q. How do you write Aufbau principle?
Q. Is 2s possible?
(ii) The second sub-shell has two subshells, i.e., 2s and 2p. Therefore, 2s orbitals are possible.
Q. Why is it called Hund’s rule?
Your Answer:The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This implies that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs.
Q. What is maximum multiplicity?
During the filling of orbitals the maximum number of unpaired electrons is achieved and hence the maximum total spin state. This is the maximum multiplicity.
Q. Is called Rule of maximum multiplicity?
Hund’s rule of maximum multiplicity It is a rule which depends on the observation of atomic spectra, which is helpful in predicting the ground state of a molecule or an atom with one or more than one open electronic shells. This rule was discovered in the year 1925 by Friedrich Hund.
Q. Why Hund’s rule is called maximum multiplicity rule?
This is because out of the various possible electronic configurations, only that configuration is correct for which the total spin value is maximum.
Q. What is the spin multiplicity of oxygen?
Figure 1: Electronic configurations 3P, 1D and 1S of the partially filled 2p orbitals of atomic oxygen. 1 The multiplicity is given by 2S+1, where S is the spin. The spin of an electron is (+/-) 1/2. oxygen is a bi-radical.
Q. What is Auf Bau principle what is Hund’s rule?
Hunds Rule According to this rule electron pairing in p, d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied. It states that: In a sublevel, each orbital is singly occupied before it is doubly occupied.
Q. How do you write Aufbau principle?
Using the Aufbau Principle
- Write a column of s orbitals from 1 to 8.
- Write a second column for the p orbitals starting at n=2.
- Write a column for the d orbitals starting at n=3.
- Write a final column for 4f and 5f.
- Read the chart by running the diagonals starting from 1s.