When there is a high number of protons, the positive charge increases. The size of the charges on the ions makes a difference in the coulombic attraction. The ions with a larger charge will attract more opposite charged ions towards itself compared to ions with a smaller charge.
Q. What is the force of attraction in a solid?
The gas particles have big distances between them. Solid – In a solid, the attractive forces keep the particles together tightly enough so that the particles do not move past each other. Their vibration is related to their kinetic energy. In the solid the particles vibrate in place.
Table of Contents
- Q. What is the force of attraction in a solid?
- Q. How do you calculate coulombic attraction?
- Q. How does distance affect coulombic attraction?
- Q. What is the force of attraction between a positive and negative charge?
- Q. Does coulombic attraction increase down a group?
- Q. How does coulombic attraction affect atomic radius?
- Q. What are the variables that affect the strength of coulombic attractions?
- Q. Which best describes the group trend for coulombic attraction?
- Q. What is the mathematical relationship between distance and attractive force?
- Q. What makes it harder to remove an electron?
- Q. Why is it difficult to remove the second electron from magnesium?
- Q. Is it hard or easy to remove an electron from magnesium?
Q. How do you calculate coulombic attraction?
Ions exhibit attractive forces for ions of opposite charge — hence the adage that “opposites attract.” The force of attraction between oppositely charged ions follows Coulomb’s law: F = k * q1 * q2 / d2, where F represents the force of attraction in Newtons, q1 and q2 represents the charges of the two ions in coulombs …
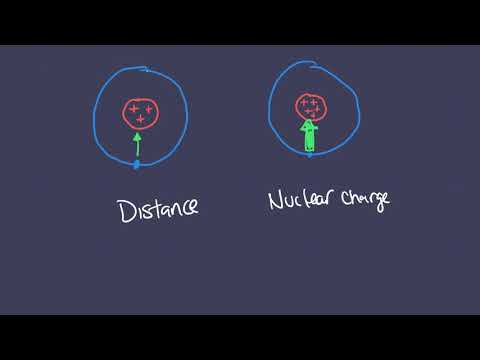
Q. How does distance affect coulombic attraction?
The farther away, the less the attraction for an atom’s electrons. The more protons, the greater the attraction for an atom’s electrons. The more electrons, the less the attraction for an atom’s electrons.
Q. What is the force of attraction between a positive and negative charge?
Definition of Coulombic Attraction Coulombic attraction is the force of attraction between positive and negative charges. It is easy to calculate the force between two charged particles using Coulomb’s law. If the charges on the particles have opposite signs, the force will be one of attraction.
Q. Does coulombic attraction increase down a group?
Some atoms have a strong coulombic attraction compared to others due to the number of protons in the nucleus. Effective nuclear charge increase more steadily going across a period (more protons but not more shells) than going down a group (more protons but also more shells).
Q. How does coulombic attraction affect atomic radius?
According to Coulomb’s Law, as the atomic number increases within a series of atoms, the nuclear attraction for electrons will also increase, thus pulling the electron(s) closer to the nucleus. Such a relationship between atomic number and atomic radius is a direct correlation. an inverse correlation.
Q. What are the variables that affect the strength of coulombic attractions?
This coulombic attraction causes electrons to orbit around the nucleus.) The strength of the coulombic attraction depends on two things: The size of the atom The total charge of the atom The larger the size of the atom, the electrons, especially the valence electrons, are further away from the nucleus.
Q. Which best describes the group trend for coulombic attraction?
Which best describes the group trend for Coulombic attraction? The distance between the outermost electrons and the nucleus is increasing.
Q. What is the mathematical relationship between distance and attractive force?
Consider the data presented in Models 1 and 3. a. Describe the mathematical relationship between the distance (d) and the attractive force (F) between protons and electrons. Force is inversely proportional to the distance squared between proton and electrons.
Q. What makes it harder to remove an electron?
It becomes harder to remove an electron when an atom has a net positive charge because the attraction that the nuclear charge exerts per electron gets larger. For example, if you have a neutral nitrogen atom, it has 7 electrons.
Q. Why is it difficult to remove the second electron from magnesium?
Magnesium has the electron configuration of 1s22s22p63s2. Electrons in lower levels feel a greater attraction to the nucleus and are more difficult to remove. Also the second level is stable octet with a full 2s and a full 2p6 orbital.
Q. Is it hard or easy to remove an electron from magnesium?
The electron being removed from a Mg atom is a 3 s electron, which is only shielded by the inner core electrons. Since there is a greater degree of electron shielding in the Al atom, it is slightly easier to remove the valence electron and its ionization energy is less than that of Mg.