An atom loses electrons to form a cation , that is a positively charged ion (and one that is attracted towards the negatively charged terminal, the cathode ). Both charge and mass have been conserved.
Q. Do nonmetals lose or gain?
Nonmetals tend to gain electrons in order to achieve a full outer shell, so they are said to have high electronegativities. Alkaline metals, for example, would find it much easier to lose electrons than gain electrons, so they are not very electronegative.
Table of Contents
- Q. Do nonmetals lose or gain?
- Q. Why do metals lose electrons when nonmetals gain?
- Q. What is the only nonmetal that can lose electrons?
- Q. Do nonmetals accept electrons?
- Q. Can a metal gain electrons?
- Q. Which element is most likely to gain electrons?
- Q. Why would a metal lose electrons?
- Q. What elements are likely to gain electrons?
- Q. Why do atoms tend to gain or lose electrons?
- Q. What happens if an atom loses or gains an electron?
- Q. What happens if an electron is lost?
- Q. Why does nitrogen have a 3 charge?
- Q. What happens when nitrogen gains 3 electrons?
- Q. How does magnesium become stable?
- Q. How many electrons does magnesium need to stable?
- Q. Is magnesium stable or unstable?
- Q. Why is magnesium flammable?
- Q. Can Magnesium be broken down by chemical means?
- Q. Why is Mg unstable?
- Q. Is hydrogen stable or unstable?
- Q. Why is Mg reactive?
Q. Why do metals lose electrons when nonmetals gain?
In regards to the octet rule, why do metals tend to lose electrons and nonmetals tend to gain electrons? Atoms of metals tend to lose their valence electrons, leaving a complete octet in the next-lowest energy level, because they generally have 4- valence electrons and it’s easier to lose.
Q. What is the only nonmetal that can lose electrons?
Hydrogen
Q. Do nonmetals accept electrons?
In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Similarly, nonmetals that have close to 8 electrons in their valence shells tend to readily accept electrons to achieve noble gas configuration.
Q. Can a metal gain electrons?
Ionic bonds form between metals and non-metals. Metals are the elements on the left side of the Periodic Table. Metals tend to lose electrons and non-metals tend to gain electrons, so in reactions involving these two groups, there is electron transfer from the metal to the non-metal.
Q. Which element is most likely to gain electrons?
Chlorine
Q. Why would a metal lose electrons?
Metal atoms lose electrons to nonmetal atoms because metals typically have relatively low ionization energies. Metals at the bottom of a group lose electrons more easily than those at the top.
Q. What elements are likely to gain electrons?
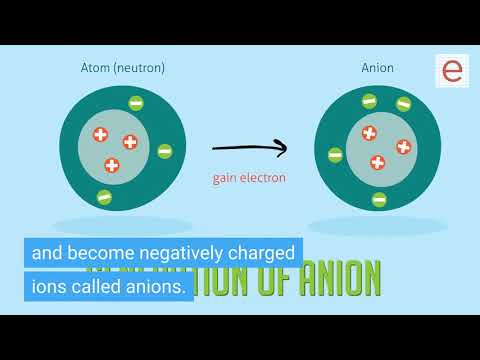
Answer: Elements that are metals tend to lose electrons and become positively charged ions called cations. Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions.
Q. Why do atoms tend to gain or lose electrons?
Atoms tend to gain or loss electrons in order to attain their nearest noble gas configuration and the completion of their octate helps them gain stability.
Q. What happens if an atom loses or gains an electron?
An atom that gains or loses an electron becomes an ion. If it gains a negative electron, it becomes a negative ion. If it loses an electron it becomes a positive ion (see page 10 for more on ions).
Q. What happens if an electron is lost?
If an atom has an equal number of protons and electrons, its net charge is 0. If it gains an extra electron, it becomes negatively charged and is known as an anion. If it loses an electron, it becomes positively charged and is known as a cation.
Q. Why does nitrogen have a 3 charge?
Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals.
Q. What happens when nitrogen gains 3 electrons?
But typically a nitrogen atom gains 3 electrons to form the nitride ion, N3− .
Q. How does magnesium become stable?
At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). It reacts directly with many elements.
Q. How many electrons does magnesium need to stable?
8 electrons
Q. Is magnesium stable or unstable?
Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei.
Q. Why is magnesium flammable?
When magnesium is in its metal form it will burn very easily in air. However, in order to start the reaction (the burning) the magnesium metal needs a source of energy. The flame provides a source of heat so that the magnesium metal atoms can overcome their activation energy.
Q. Can Magnesium be broken down by chemical means?
Which substance can be decomposed by a chemical change? Reason: when it said a substance, think of the matter chart. Substances can be broken down to a compounds or elements. Beryllium, Boron, and Magnesium are elements and Methanol is a compound which can be chemically change.
Q. Why is Mg unstable?
What is Mg+ ion unstable? Mg+ ion unstable because Mg+ ion has still the tendency to lose another electron to form Mg2+ ion which has the stable noble gas configuration of the nearest inert gas (Neon). Alkali metal has one electron each in the valence subshell of their atoms.
Q. Is hydrogen stable or unstable?
Hydrogen only has one electron in its lowest energy level. This is a very unstable arrangement, and hydrogen gas undergoes a variety of reactions so as to reach a stable electron configuration where its energy level is either empty of electrons, or filled with electrons.
Q. Why is Mg reactive?
So now you can see that from the periodic table Magnesium is more reactive than Zinc and Aluminum. Because magnesium loses its electrons easily than zinc and aluminum, other elements and compounds like oxygen, water and the halogens reacts vigorously with atoms that loses electron easily.