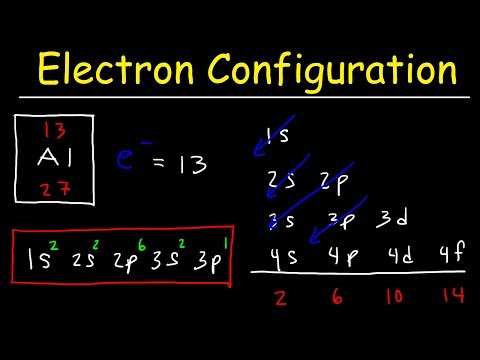
It means there are 2- electrons in first energy level s-subshell and 2-electrons 2nd energy level s- sub shell and 2-electrons in 2nd energy level p-sub shell. Energy level. Electrons. s – orbital.
Q. What element is 1s2 2s2 2p3?
Nitrogen
Table of Contents
- Q. What element is 1s2 2s2 2p3?
- Q. What does the S mean in 1s2?
- Q. What element or ion has the electron configuration 1s 22s 22p 6?
- Q. Are K+ and Ar Isoelectronic?
- Q. What element is best represented by the electron configuration?
- Q. What is the correct electron configuration for iodine?
- Q. Which electrons are removed first when making a cation?
- Q. What is the electron configuration of SN 4?
- Q. What is the name of Sn4+?
- Q. Which of the following electron configuration is not possible?
- Q. Which of the following is the electron configuration of a neutral K atom?
- Q. Which element has a 5+ ion and an electronic configuration of 1s2 2s2 2p6 3s2 3p6 3d10?
- Q. How many electrons does K have?
Q. What does the S mean in 1s2?
The number in superscript is the number of electrons in a sub-shell. Each sub-shell can hold only a certain number of electrons. The s sub-shell can hold no more than 2 electrons, the p sub-shell can hold 6, the d sub-shell can hold 10 and the f sub-shell can hold as many as 14.
Q. What element or ion has the electron configuration 1s 22s 22p 6?
element Silicon
Q. Are K+ and Ar Isoelectronic?
And we can see that the potassium ion, K+, has the same electronic configuration as the chloride ion, Cl-, and the same electronic configuration as an atom of argon, Ar. Therefore, Ar, Cl-, and K+ are said to be isoelectronic species.
Q. What element is best represented by the electron configuration?
The answer must be neon because the number of electrons is 2 + 2 + 6 = 10. Neon has an atomic number of 10. Therefore, the answer is neon.
Q. What is the correct electron configuration for iodine?
[Kr] 4d10 5s2 5p5
Q. Which electrons are removed first when making a cation?
The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s22s22p63s23p64s2.
Q. What is the electron configuration of SN 4?
Tin(IV): [Kr] 4d^10 because the 4 electrons are taken away from the highest energy orbitals first.
Q. What is the name of Sn4+?
Tin
Q. Which of the following electron configuration is not possible?
– Since the third energy level can have s, p, and d orbitals only, the electronic configuration of 3f12 is not possible.
Q. Which of the following is the electron configuration of a neutral K atom?
In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s23p64s1 .
Q. Which element has a 5+ ion and an electronic configuration of 1s2 2s2 2p6 3s2 3p6 3d10?
One is taken from the 4s sub-shell, the other from the 3d sub-shell. So the electron configuration of Cu2+ is: 1s2 2s2 2p6 3s2 3p6 3d9.
Q. How many electrons does K have?
19 electrons