A way of representing atoms or molecules by showing electrons as dots surrounding the element symbol. One bond is represented as two electrons.
Q. What is an electron dot formula?
Electron dot formula shows the number of valence electrons for that element with the help of dots. For example, the elements in group IA of the chemical periodic table have 1 valence electron. In chemistry electron dot formula has its own importance.
Table of Contents
- Q. What is an electron dot formula?
- Q. What is the electron dot structure of HCl?
- Q. What are the two rules when writing Lewis dot diagram?
- Q. What are the dots in Lewis dot structure?
- Q. What shape is SiCl4?
- Q. Why SiCl4 is a Lewis acid?
- Q. Is SiCl4 an electrophile?
- Q. Can SiCl4 act as Lewis acid?
- Q. Is CCl4 acidic or basic?
- Q. What is the pH of CCl4?
- Q. Why ccl4 is insoluble in water sicl4 is soluble?
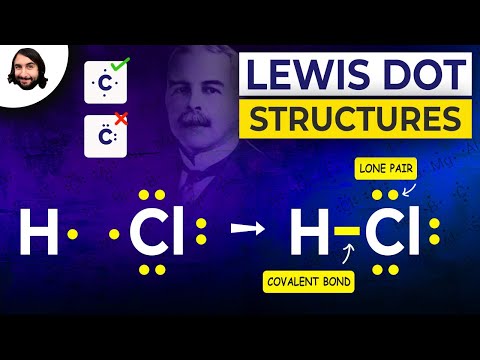
Q. What is the electron dot structure of HCl?
If we wanted to show the Lewis structure of HCl, we would draw the following: We can see that the covalent bond consists of two electrons between the H and the Cl. The H has a full outer shell of two electrons and the chlorine has a full outer shell of eight electrons.
Q. What are the two rules when writing Lewis dot diagram?
Step 1: Determine the total number of valence electrons. Step 2: Write the skeleton structure of the molecule. Step 3: Use two valence electrons to form each bond in the skeleton structure. Step 4: Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons.
Q. What are the dots in Lewis dot structure?
The dots in a Lewis dot structure represent an atom’s valence electrons and the placement of the dots indicate how the electrons are distributed in a molecule. The number of valence electrons corresponds to the column numbers.
Q. What shape is SiCl4?
tetrahedral shape
Q. Why SiCl4 is a Lewis acid?
Now, sicl4 is able to keep greater than 8 electrons in its outermost orbit. So it takes electron pairs from other compounds which have more electron density. It can also increase it’s coordination number. So it works as a Lewis acid.
Q. Is SiCl4 an electrophile?
Silicon tetrachloride is a classic electrophile in its reactivity.
Q. Can SiCl4 act as Lewis acid?
CCl4 does not act as a Lewis acid while SiCl4 and SnCl4 act as Lewis acids.
Q. Is CCl4 acidic or basic?
Carbon tetrachloride is NEITHER an acid nor a base.
Q. What is the pH of CCl4?
6-10
Q. Why ccl4 is insoluble in water sicl4 is soluble?
CCl4 is highly non polar molecule. So it doesnot dissolve in water. SiCl4 is a polar molecule. So it is dissolved in water.