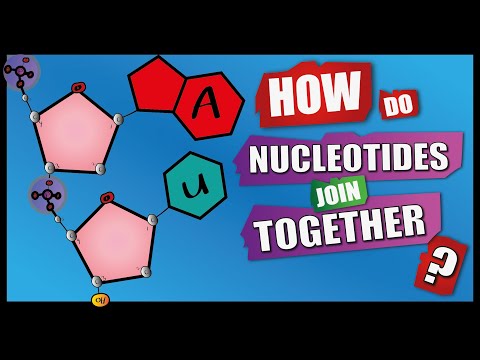
Bases are held together by hydrogen bonds, and the DNA backbone is held together by phosphodiester bonds.
Q. How are double helix bonds held?
Double Helix Attached to each sugar is one of four bases: adenine (A), cytosine (C), guanine (G), or thymine (T). The two strands are held together by bonds between the bases, adenine forming a base pair with thymine, and cytosine forming a base pair with guanine.
Table of Contents
- Q. How are double helix bonds held?
- Q. What bond keeps the double helix together?
- Q. What type of bond holds the two strands together in double stranded DNA?
- Q. What kind of bonds hold two polynucleotides together what holds the double helix together )? Are they strong?
- Q. Why are the two strands of DNA attracted to each other?
- Q. What stabilizes the DNA double helix?
- Q. Why is the DNA double helix so stable?
- Q. Which DNA is most stable?
- Q. Why is Double Helix important?
- Q. Does RNA have double helix?
- Q. Is DNA really a double helix?
- Q. How did the double helix impact society?
- Q. Who really discovered the DNA helix?
- Q. Is genetic mutation good or bad?
- Q. How was the double helix of DNA discovered?
- Q. What did Watson and Crick get wrong?
- Q. Did Watson and Crick steal?
- Q. Who did Watson and Crick steal from?
- Q. What did Watson and Crick discover about DNA?
- Q. Who did Watson Crick and Wilkins steal the idea of the double helix from?
- Q. Has DNA been photographed?
- Q. Why is it called Photo 51?
- Q. What did Rosalind Franklin find out about DNA?
- Q. Why was Rosalind Franklin called the Dark Lady of DNA?
- Q. Why didnt Rosalind Franklin get credit?
- Q. What is Rosalind Franklin’s legacy?
- Q. What type of force holds the double helix of DNA together?
- Q. How many hydrogen bonds are represented in this double helix?
- Q. What are the 2 strands of DNA held together?
- Q. Which compound has the strongest hydrogen bonding at STP?
- Q. Which compound has the strongest intermolecular forces?
- Q. How many types of hydrogen bond are there?
- Q. Which substance has the strongest intermolecular forces?
- Q. What are the 3 intermolecular forces from weakest to strongest?
- Q. Which is stronger water or ethanol?
- Q. Why are hydrogen bonds so strong?
Q. What bond keeps the double helix together?
hydrogen bonds
Q. What type of bond holds the two strands together in double stranded DNA?
hydrogen bonding
Q. What kind of bonds hold two polynucleotides together what holds the double helix together )? Are they strong?
Hydrogen bonds occur between the two strands and involve a base from one strand with a base from the second in complementary pairing. These hydrogen bonds are individually weak but collectively quite strong.
Q. Why are the two strands of DNA attracted to each other?
The two strands bind through the bonding of the bases of each nucleotide i.e. the bases from one strand bond to the bases of the second strand of DNA. This makes the two chains run anti-parallel to each other and gives the DNA the spiraling structure that makes the double helix.
Q. What stabilizes the DNA double helix?
The structure of the DNA helix is stabilized by van der Waals forces, hydrogen bonds between complementary organic bases (a base pair), and hydrophobic interactions between the nitrogenous bases and the surrounding sheath of water.
Q. Why is the DNA double helix so stable?
The main bonding in DNA which renders the double helix structure so stable is that of hydrogen bonds. Between the complementary base pairs, hydrogen bonds connect the two strands of the helix. There are 3 H bonds between Guanine and Cytosine and 2 between Adenine and Thymine.
Q. Which DNA is most stable?
B form
Q. Why is Double Helix important?
The double-helix shape allows for DNA replication and protein synthesis to occur. In these processes, the twisted DNA unwinds and opens to allow a copy of the DNA to be made. In DNA replication, the double helix unwinds and each separated strand is used to synthesize a new strand.
Q. Does RNA have double helix?
Although usually single-stranded, some RNA sequences have the ability to form a double helix, much like DNA. Gehring said identifying the double-helical RNA will have interesting applications for research in biological nanomaterials and supramolecular chemistry.
Q. Is DNA really a double helix?
Each DNA molecule is actually a pair of strands wound together, forming a double helix. To make a protein from a gene, a cell must separate the strands and read their sequence. To reproduce, a cell must rip the strands apart and build new counterparts for each one.
Q. How did the double helix impact society?
The rapid growth in our understanding over the past 60 years, including the delivery of genomes for a range of species including humans, has affected all of us at some level. This knowledge has brought improved medical treatments, new drugs and better disease diagnosis.
Q. Who really discovered the DNA helix?
Francis Crick
Q. Is genetic mutation good or bad?
Mutational effects can be beneficial, harmful, or neutral, depending on their context or location. Most non-neutral mutations are deleterious. In general, the more base pairs that are affected by a mutation, the larger the effect of the mutation, and the larger the mutation’s probability of being deleterious.
Q. How was the double helix of DNA discovered?
Created by Rosalind Franklin using a technique called X-ray crystallography, it revealed the helical shape of the DNA molecule. Watson and Crick realized that DNA was made up of two chains of nucleotide pairs that encode the genetic information for all living things.
Q. What did Watson and Crick get wrong?
Watson and Crick’s model erroneously placed the bases on the outside of the DNA molecule with the phosphates, bound by magnesium or calcium ions, inside. One of the key characteristics of science is that it relies on evidence.
Q. Did Watson and Crick steal?
One claim was that during the race to uncover the structure of DNA, Jim Watson and Francis Crick either stole Rosalind Franklin’s data, or ‘forgot’ to credit her. Neither suggestion is true. The model the Cambridge duo put forward did not simply describe the DNA molecule as a double helix.
Q. Who did Watson and Crick steal from?
Franklin is best known for her work on the X-ray diffraction images of DNA while at King’s College London, particularly Photo 51, taken by her student Raymond Gosling, which led to the discovery of the DNA double helix for which Francis Crick, James Watson, and Maurice Wilkins shared the Nobel Prize in Physiology or …
Q. What did Watson and Crick discover about DNA?
On February 28, 1953, Cambridge University scientists James D. Watson and Francis H.C. Crick announce that they have determined the double-helix structure of DNA, the molecule containing human genes.
Q. Who did Watson Crick and Wilkins steal the idea of the double helix from?
Rosalind
Q. Has DNA been photographed?
On 6 May 1952, at King´s College London in London, England, Rosalind Franklin photographed her fifty-first X-ray diffraction pattern of deoxyribosenucleic acid, or DNA.
Q. Why is it called Photo 51?
The image was tagged “photo 51” because it was the 51st diffraction photograph that Franklin and Gosling had taken. It was critical evidence in identifying the structure of DNA.
Q. What did Rosalind Franklin find out about DNA?
Rosalind Franklin discovered the density of DNA and, more importantly, established that the molecule existed in a helical conformation. Her work to make clearer X-ray patterns of DNA molecules laid the foundation for James Watson and Francis Crick’s suggestion that DNA is a double-helix polymer in 1953.
Q. Why was Rosalind Franklin called the Dark Lady of DNA?
Franklin’s biographer, Brenda Maddox, called her “the Dark Lady of DNA”, based on a disparaging reference to Franklin by one of her coworkers, and also because although her work on DNA was crucial to the discovery of its structure, her contribution to that discovery is little known.
Q. Why didnt Rosalind Franklin get credit?
Franklin, whose lab produced the photograph that helped unravel the mystery of DNA, received no credit for her role until after her death. At the time of her death, she was working on the molecular structure of viruses with her colleague Aaron Klug, who received a Nobel Prize for the work in 1982.
Q. What is Rosalind Franklin’s legacy?
Rosalind Franklin’s short scientific carrier produced brilliant contri- butions to the structure of carbon, DNA, and helical and spherical vi- ruses. At 30, she was a recognized authority who switched from car- bon to DNA research and, a few years later, to nucleic-acid-protein complexes known as viruses.
Q. What type of force holds the double helix of DNA together?
Hydrogen bonds
Q. How many hydrogen bonds are represented in this double helix?
In the structures for the complementary base pairs given in the graphic on the left, notice that the thymine – adenine pair interacts through two hydrogen bonds represented as (T=A) and that the cytosine-guanine pair interacts through three hydrogen bonds represented as (C=G).
Q. What are the 2 strands of DNA held together?
Base Pair Attached to each sugar is one of four bases–adenine (A), cytosine (C), guanine (G), or thymine (T). The two strands are held together by hydrogen bonds between the bases, with adenine forming a base pair with thymine, and cytosine forming a base pair with guanine.
Q. Which compound has the strongest hydrogen bonding at STP?
The strongest hydrogen bond type is the one involving a bond between oxygen, nitrogen and fluorine, with a hydrogen atom. Since in the choices, only water shows a O-H bond, therefore this is the strongest hydrogen bonding. The answer is water.
Q. Which compound has the strongest intermolecular forces?
HF (boiling point = 19.4 degrees Celsius) has the strongest intermolecular forces.
Q. How many types of hydrogen bond are there?
two types
Q. Which substance has the strongest intermolecular forces?
Q. What are the 3 intermolecular forces from weakest to strongest?
There are three different types of intermolecular forces in terms of strength. They are (strongest to weakest) hydrogen bonding, dipole-dipole and Van der Waals’ forces.
Q. Which is stronger water or ethanol?
As a result, it is more difficult to deform the surface of water than the surface of ethyl alcohol. Therefore, since water molecules on a liquid surface are harder to push down on the surface tension is higher for water than for ethyl alcohol.
Q. Why are hydrogen bonds so strong?
Hydrogen bonding is so strong among dipole-dipole interactions because it itself is a dipole-dipole interaction with one of the strongest possible electrostatic attractions. Remember that hydrogen bonding cannot occur unless hydrogen is covalently bonded to either oxygen, nitrogen, or fluorine.