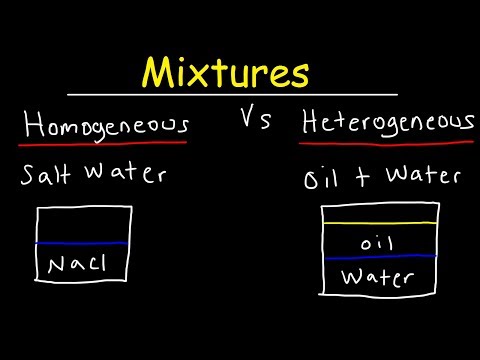
Types of Mixtures There are two main categories of mixtures: homogeneous mixtures and heterogeneous mixtures. In a homogenous mixture all the substances are evenly distributed throughout the mixture (salt water, air, blood).
Q. How matter is useful to us?
Matter is important for us because it has mass and it occupies space therefore all things that we can touch and see is part of matter.
Table of Contents
- Q. How matter is useful to us?
- Q. What is matter give two examples?
- Q. What are three different matters?
- Q. What are the 2 types of matter?
- Q. What are the uses of mixtures?
- Q. What is the types of mixtures?
- Q. What are five mixtures?
- Q. What are the three properties of mixtures?
- Q. What are mixtures and solutions?
- Q. How do you teach mixtures and solutions?
- Q. What are examples of mixtures and solutions?
- Q. How mixture can affect our daily life?
- Q. Are all mixture useful to us?
- Q. What are three examples Solutions?
- Q. What elements do we use everyday?
- Q. Which element is highest in human body?
- Q. What are the five elements of life?
Q. What is matter give two examples?
Matter is a substance that has inertia and occupies physical space. Examples :-solids, liquids, gases, plasma and Bose-Einstein condensates.
Q. What are three different matters?
They are very compressible (particles are widely spaced). There are three states of matter: solid; liquid and gas.
Q. What are the 2 types of matter?
Classifying Matter Matter can be classified into several categories. Two broad categories are mixtures and pure substances. A pure substance has a constant composition. All specimens of a pure substance have exactly the same makeup and properties.
Q. What are the uses of mixtures?
1: Mixtures are useful when you want to combine elements of multiple materials into one product. I.E. you want to rehydrate AND replenish electrolytes so you mix salt in water (or buy Brondo.) 2: Mixtures allow you to dilute a pure ingredient into a product with less ingredient per unit volume.
Q. What is the types of mixtures?
There are two types of mixtures: heterogeneous and homogeneous. Heterogeneous mixtures have visually distinguishable components, while homogeneous mixtures appear uniform throughout. The most common type of homogenous mixture is a solution, which can be a solid, liquid, or gas.
Q. What are five mixtures?
Here are a few more examples:
- Smoke and fog (Smog)
- Dirt and water (Mud)
- Sand, water and gravel (Cement)
- Water and salt (Sea water)
- Potassium nitrate, sulfur, and carbon (Gunpowder)
- Oxygen and water (Sea foam)
- Petroleum, hydrocarbons, and fuel additives (Gasoline)
Q. What are the three properties of mixtures?
Mixtures can be classified on the basis of particle size into three different types: solutions, suspensions, and colloids. The components of a mixture retain their own physical properties. These properties can be used to separate the components by filtering, boiling, or other physical processes.
Q. What are mixtures and solutions?
Mixtures are materials that contain two or more chemical substances dispersed among each other (mixed together). Solutions are homogenous mixtures: particles of one substance (the solute) are mixed together with the particles of another substance (the solvent) – eg salty water.
Q. How do you teach mixtures and solutions?
Teacher demonstration: Set up three glasses of water.
- Add pebbles of sand to the first glass. Stir the water.
- Add a teaspoon of salt to the second glass. Stir the water until the salt disappears.
- Ask students if two liquids will form a mixture or a solution. Then add some vegetable oil to the third glass and stir.
Q. What are examples of mixtures and solutions?
Solution: a homogeneous mixture of two or more substances. Example: water, sugar, flavor mixture (Coke). The substances are physically combined, not chemically combined or bonded to each other. Solvent: usually the substance in the greater amount.
Q. How mixture can affect our daily life?
Answer. They mixed large amounts of sugar into the concrete, slowing down the setting process, and allowing them time to clear up the spill. Mixtures and solutions are a common occurrence in our everyday lives. They are the air we breathe, the food and drink we consume and the fabrics we wear.
Q. Are all mixture useful to us?
Not necessarily, mixture may comprise of one or more form of matter. Separation of mixture into its individual components may be useful it depends upon whether component we are separating is of use to us or not.
Q. What are three examples Solutions?
Some examples of solutions are salt water, rubbing alcohol, and sugar dissolved in water. When you look closely, upon mixing salt with water, you can’t see the salt particles anymore, making this a homogeneous mixture.
Q. What elements do we use everyday?
The most important elements that we use in everyday life include carbon, hydrogen, oxygen, with smaller amounts of things like chlorine, sulfur, calcium, iron, phosphorus,nitrogen, sodium, and potassium. Apart from these, other elements include magnesium, zinc, neon, and helium are also in our daily existence.
Q. Which element is highest in human body?
Oxygen
Q. What are the five elements of life?
Everything in nature is made up of five basic elements: earth, water, fire, air, and space.