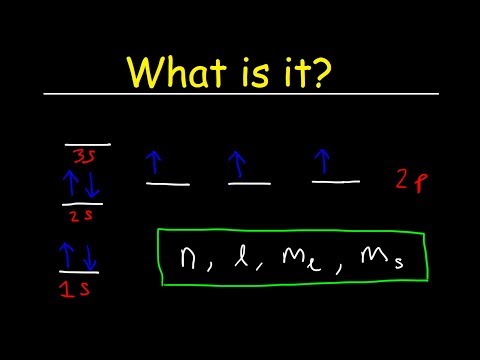
When assigning electrons to orbitals, we must follow a set of three rules: the Aufbau Principle, the Pauli-Exclusion Principle, and Hund’s Rule.
Q. How do I remember Pauli exclusion principle?
I remember them by Aufbau is A-Z: fill lowest to highest energy, Hund’s rule is half-filled orbitals first, and Pauli exclusion involves exclusion: no two electrons can have the same four quantum numbers. That’s really helpful!
Table of Contents
- Q. How do I remember Pauli exclusion principle?
- Q. What is the basic concept and different between Pauli exclusion principle Aufbau principle and Hund’s rule of filling Orbital?
- Q. Why is the Pauli exclusion principle the key to understanding the periodic table?
- Q. Which of the following orbital diagrams are both Pauli exclusion principle and Hund’s rule violated?
- Q. Which of the following is violation of Pauli’s exclusion principle?
- Q. Which rule is violated by the electron configuration below?
- Q. Which rule does this violate 1s1 2s2?
- Q. Which rule is violated in the electronic configuration 1s2 2s2 2p6 3s2 3p6 4s0 3d1 and how?
- Q. Why are 3d orbitals filled after 4s?
Q. What is the basic concept and different between Pauli exclusion principle Aufbau principle and Hund’s rule of filling Orbital?
In simple terms, the Aufbau principle means fill the orbitals from bottom to top. In simple terms, Hund’s rule requires single occupancy before pairing. Pauli Exclusion Principle. No two electrons in a atom can have an identical set of four quantum numbers.
Q. Why is the Pauli exclusion principle the key to understanding the periodic table?
Why Is the Pauli Exclusion Principle Important? The Pauli exclusion principle informs electron configuration and the way atoms are classified in the periodic table of elements. Ground state, or lowest energy levels in an atom can fill up, forcing any additional electrons to higher energy levels.
Q. Which of the following orbital diagrams are both Pauli exclusion principle and Hund’s rule violated?
In option {d} orbital diagrams both Pauli’s exclusion principle and Hund’s rule violated.
Q. Which of the following is violation of Pauli’s exclusion principle?
The 1s and 2s subshells for beryllium atoms can hold only two electrons, and when filled, the electrons must have opposite spins or have the same four quantum numbers. Thus violating the Pauli Exclusion Principle.
Q. Which rule is violated by the electron configuration below?
Aufbau Principle
Q. Which rule does this violate 1s1 2s2?
Aufbau principle is violated in this electronic configuration.
Q. Which rule is violated in the electronic configuration 1s2 2s2 2p6 3s2 3p6 4s0 3d1 and how?
Ans: Aufbau principle is violated in this electronic configuration because according to Aufbau principle, Electron enters into orbital of lower energy.
Q. Why are 3d orbitals filled after 4s?
We say that the 4s orbitals have a lower energy than the 3d, and so the 4s orbitals are filled first. The electrons lost first will come from the highest energy level, furthest from the influence of the nucleus. So the 4s orbital must have a higher energy than the 3d orbitals.