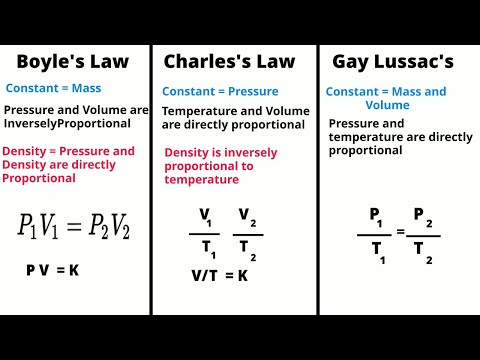
The gas laws consist of three primary laws: Charles’ Law, Boyle’s Law and Avogadro’s Law (all of which will later combine into the General Gas Equation and Ideal Gas Law).
Q. How do you explain Charles Law?
Charles’s law, a statement that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant. It is a special case of the general gas law and can be derived from the kinetic theory of gases under the assumption of a perfect (ideal) gas.
Table of Contents
- Q. How do you explain Charles Law?
- Q. What is Charles Law quizlet?
- Q. What is the ideal gas law quizlet?
- Q. What does Avogadro’s law state?
- Q. Why is Avogadro’s law important?
- Q. What are the main applications of Avogadro’s law?
- Q. What is Avogadro’s number and what is it used for?
- Q. What is Avogadro gas law formula?
- Q. What is real gas law?
- Q. What is ideal gas equation derive it?
- Q. How will you define mole?
- Q. What is the importance of a mole?
- Q. How are moles used in everyday life?
- Q. How many is a mole?
- Q. What is a mole Class 9?
- Q. Why is a mole 6.022 x10 23?
- Q. What is the value of 6.022 into 10 to the power 23?
- Q. What is the relationship between Avogadro’s number and one mole?
- Q. What is the relationship between Avogadro’s number and one mole quizlet?
- Q. How Avogadro’s number was found?
Q. What is Charles Law quizlet?
Charles law. States that the volume of a given mass of gas is directly proportional to its kelvin temperature at constant pressure. Gay-lussac’s law. States that the pressure of a fixed mass of gass varies directly with the Kelvin temperature when the volume remains constant.
Q. What is the ideal gas law quizlet?
Ideal Gas Law. gives the relation ship between the pressure, volume, temperature, and number of moles for a sample of gas. (The Ideal Gas Law is derived from the Combined Gas Law and Avogadro’s Principle.) Constant. This constant is called the ideal gas constant and is given the symbol R.
Q. What does Avogadro’s law state?
Avogadro’s law, a statement that under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of molecules. The law is approximately valid for real gases at sufficiently low pressures and high temperatures.
Q. Why is Avogadro’s law important?
Avogadro’s law investigates the relationship between the amount of gas (n) and volume (v). It’s a direct relationship, meaning the volume of a gas is directly propotional to the number of moles the gas sample present. The law is important because helps us save time and money in the long-run.
Q. What are the main applications of Avogadro’s law?
(i) It determines the molecule formula of a gas. (ii) It determines atomicity of gases. (iii) It explains Gay-Lussac’s law of combining volumes. (iv) It establishes the relation between molecular weight and vapour density of a gas.
Q. What is Avogadro’s number and what is it used for?
Overview of how Avogadro’s number is used to measure the number of units of any substance. Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 1023.
Q. What is Avogadro gas law formula?
The number of molecules or atoms in a specific volume of ideal gas is independent of size or the gas’ molar mass. Avogadro’s Law is stated mathematically as follows: Vn=k, where V is the volume of the gas, n is the number of moles of the gas, and k is a proportionality constant.
Q. What is real gas law?
The relationship between pressure and volume for a gas is usually expressed as the real gas law: (2.5.17) in which v is the molar volume, z is the gas compressibility factor, R is the universal gas constant, and T is temperature.
Q. What is ideal gas equation derive it?
The most common form of this equation is since PV= K and V/T =k then. PV/T = constant. Thus, the Ideal Gas Equation is given as. PV = nRT. where P= pressure of the gas; V=volume of the gas; n= Number of Moles; T=Absolute temperature; R=Ideal Gas constant also known as Boltzmann Constant = 0.082057 L atm K-1 mol-1.
Q. How will you define mole?
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
Q. What is the importance of a mole?
The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts. Atoms, molecules and formula units are very small and very difficult to work with usually. However, the mole allows a chemist to work with amounts large enough to use.
Q. How are moles used in everyday life?
In chemistry, the mole is a unit used to talk about atoms. It is similar to other units we use everyday. For example, you might walk into the local doughnut shop and order a dozen doughnuts. In doing so, you know that you will get 12 of these snacks and the clerk knows to give you 12.
Q. How many is a mole?
6.02214179
Q. What is a mole Class 9?
Summary. Mole: Mole is the measurement in chemistry. It is used to express the amount of a chemical substance. One mole is defined as the amount of substance of a system which contains as many entities like, atoms, molecules and ions as there are atoms in 12 grams of carbon – 12″.
Q. Why is a mole 6.022 x10 23?
The mole (abbreviated mol) is the SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×1023 elementary entities of the substance.
Q. What is the value of 6.022 into 10 to the power 23?
Answer: Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10^23.
Q. What is the relationship between Avogadro’s number and one mole?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan.
Q. What is the relationship between Avogadro’s number and one mole quizlet?
Avogadro’s number is the number of representative particles in 1 mole. The mass of 6.02 x 10^23 particles of a representative of a substance is the molar mass of the substance. Compare the number of particles and the mass of 1 mole of each.
Q. How Avogadro’s number was found?
French physicist Jean Baptiste Perrin used the term Avogadro’s number for the first time while explaining Brownian motion. The value of Avogadro’s number was obtained by dividing the charge of a mole of electrons by the charge of a single electron which is equal to 6.02214154 x 1023 particles per mole.