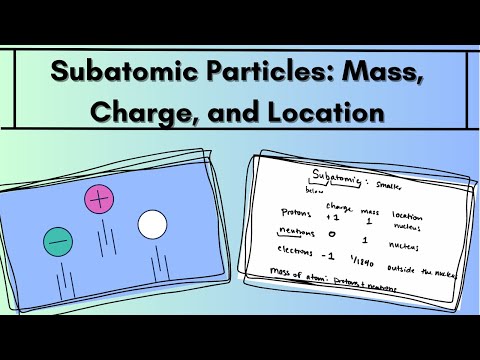
What are the charges and relative masses of the three main subatomic particles? Electrons are negatively charged particles and its mass is 1/1840 the mass of a hydrogen atom. Protons are positively charged particles. Each proton has a mass about 1840 times that of an electron.
Q. What are the subatomic particles in order of mass?
increasing mass: neutron, electron, and proton.
Table of Contents
- Q. What are the subatomic particles in order of mass?
- Q. What is the mass of each of the 3 subatomic particles?
- Q. How do you find the mass number of subatomic particles?
- Q. Do subatomic particles have mass?
- Q. How do you calculate mass number?
- Q. What is a subatomic number?
- Q. What is the mass of an electron answer?
- Q. Which subatomic particle has no mass?
- Q. What is the mass of a subatomic particle?
- Q. What is the mass and charge of a proton?
- Q. How are the masses of a particle determined?
- Q. How are scientists able to identify subatomic particles?
Q. What is the mass of each of the 3 subatomic particles?
The Three Major Subatomic Particles
| Name | Symbol | Mass (g) |
|---|---|---|
| Proton | P+ | 1.673 x 10-24 |
| Neutron | n0 | 1.675 x 10-24 |
| Electron | e– | 9.109 x 10-28 |
Q. How do you find the mass number of subatomic particles?
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Q. Do subatomic particles have mass?
Masses for the three subatomic particles can be expressed in amu (atomic mass units) or grams. For simplicity, we will use the amu unit for the three subatomics. Both neutrons and protons are assigned as having masses of 1 amu each. In contrast, the electron has a negligible mass of .
Q. How do you calculate mass number?
The mass number of an atom is calculated by adding together the number of protons and neutrons that are found within that atom. The number of neutrons is given, but the number of protons must be determined from the atomic number for the element.
Q. What is a subatomic number?
Atoms are made up of protons, neutrons and electrons, also known as subatomic particles. Because an atom is neutral, the number of electrons is equal to the number of protons. Electrons circle the nucleus, which is made up of protons and neutrons.
Q. What is the mass of an electron answer?
The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton.
Q. Which subatomic particle has no mass?
electron
The subatomic particle with almost no mass is the electron. The approximate mass of the electron is 1/1840th of an atomic mass unit.
Q. What is the mass of a subatomic particle?
Subatomic Particles. An atomic mass unit is defined as 1/12 the mass of a neutral carbon atom. Since carbon has exactly 6 protons and 6 neutrons, an atomic mass unit is equal the mass of one proton or one neutron. Since the electron is so small compared to the proton and neutron we can say it has of 0 amu.
Q. What is the mass and charge of a proton?
Atoms are made up of three subatomic particles, namely, protons, neutrons and electrons All these subatomic particles have a definite mass and charge Mass of a proton is 1.67 × 10−27 Kg and its charge is 1.6 × 10−19 Coulomb. Proton is a positively charged particle
Q. How are the masses of a particle determined?
Particle masses are measured in a great variety of ways. If they are big and heavy enough, you can put them on a scale. Subatomic particles have to be measured much more carefully than that. Typically, subatomic particle masses are determined by the relationship between their energy and their momentum.
Q. How are scientists able to identify subatomic particles?
Particle accelerators accelerate subatomic particles to nearly the speed of light and smash them into each other. Scientists gather data from these great collisions and try to identify other smaller subatomic particles. The newest subatomic particle to be identified is the Higgs Boson.