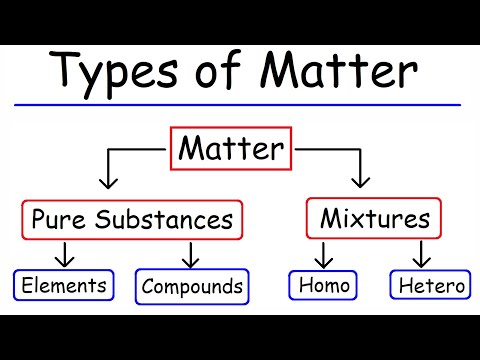
Classifying Matter Matter can be classified into several categories. Two broad categories are mixtures and pure substances. A pure substance has a constant composition. All specimens of a pure substance have exactly the same makeup and properties.
Q. What intermolecular forces are present in CH3Cl?
1. Which intermolecular forces are present in CH3Cl(s)? C so dipole-dipole forces will be present.
Table of Contents
- Q. What intermolecular forces are present in CH3Cl?
- Q. Is CH3Cl dipole-dipole and dispersion?
- Q. In which state of matter are the attractive forces the weakest?
- Q. What is the relationship between intermolecular forces and vapor pressure?
- Q. What is matter and classification of matter?
- Q. What are the 2 classes of matter Brainly?
- Q. What are the two 2 classes of matter answers?
- Q. Which is the true regarding air?
- Q. What is the physical property of matter?
- Q. What are 10 physical properties of matter?
- Q. What are the two most important properties of matter?
- Q. What are the 3 properties of matter?
Q. Is CH3Cl dipole-dipole and dispersion?
Step 4: The central element is bonded to different elements. Furthermore, the dipole moments present in CH3Cl don’t cancel out; this makes CH3Cl a polar covalent compound. This means CH3Cl exhibits dipole-dipole interaction. Also, dispersion forces which occur in all compounds.
Q. In which state of matter are the attractive forces the weakest?
Molecular solids
Q. What is the relationship between intermolecular forces and vapor pressure?
A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower the rate of evaporation and the lower the vapor pressure.
Q. What is matter and classification of matter?
Matter can be classified according to physical and chemical properties. Matter is anything that occupies space and has mass. The three states of matter are solid, liquid, and gas. Extensive properties depend on the amount of material and include mass and volume.
Q. What are the 2 classes of matter Brainly?
Answer. Answer: Matter can be broken down into two categories: pure substances and mixtures.
Q. What are the two 2 classes of matter answers?
Classifying Matter. We can classify matter into several categories. Two broad categories are mixtures and pure substances. A pure substance has a constant composition.
Q. Which is the true regarding air?
Answer Expert Verified Air is 78% nitrogen, 21% oxygen, 1% argon, and some neon, hydrogen and increasing carbon dioxide.
Q. What is the physical property of matter?
A physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance. Physical properties include color, density, hardness, and melting and boiling points.
Q. What are 10 physical properties of matter?
Physical properties include: appearance, texture, color, odor, melting point, boiling point, density, solubility, polarity, and many others.
Q. What are the two most important properties of matter?
Matter can be defined or described as anything that takes up space, and it is composed of miniscule particles called atoms. It must display the two properties of mass and volume.
Q. What are the 3 properties of matter?
The three basic properties of matter are volume, mass, and shape.