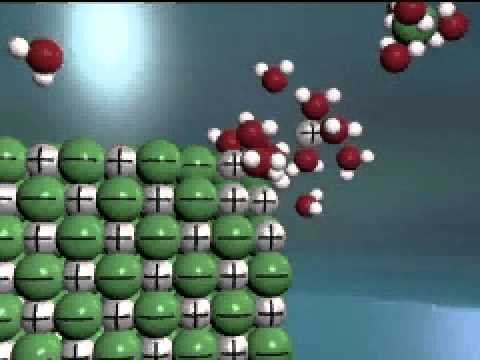
It takes just slightly more energy to separate the ions from one another than is released from the water molecules surrounding the ions. This means just slightly more energy must be put into the solution than is released back into the solution; therefore dissolving table salt in water is endothermic.
Q. When ammonium chloride is dissolved in water the solution becomes colder?
Answer. when ammonium chloride dissolves in water, the temperature falls because the ammonium chloride is pulling heat in from its surroundings (the water), causing thetemperature to fall. Thus, the energy change is endothermic.so when dissolved in water the solution becomes colder.
Table of Contents
- Q. When ammonium chloride is dissolved in water the solution becomes colder?
- Q. Is nh4cl and water exothermic?
- Q. Is NH4Cl h20?
- Q. Is NH4Cl exothermic or endothermic?
- Q. Is the heat of solution of LiCl exothermic or endothermic?
- Q. Is calcium chloride exothermic or endothermic?
- Q. Is nac2h3o2 endothermic or exothermic?
- Q. Is calcium and water endothermic or exothermic?
Q. Is nh4cl and water exothermic?
The same thing happens when ammonium chloride is dissolved in water. The endothermic separation of the ions will dictate the overall reaction, since the exothermic hydration of the ions will gfive off less energy. As a result, dissolving ammonium chloride in water will be an endothermic process.
Q. Is NH4Cl h20?
Ammonium chloride, also known as Sal ammoniac, is a compound of ammonia (NH3) and chlorine (Cl). It is denoted by the symbol NH4Cl and is in solid crystalline form in nature. This compound is a water-soluble salt of ammonia, and aqueous ammonium chloride is slightly acidic.
Q. Is NH4Cl exothermic or endothermic?
The formation of ammonium chloride from ammonia and hydrogen chloride is exothermic (heat released or heat given out to the surroundings – the cooler parts of the test tube). This means if the direction of chemical change is reversed, the energy change is also reversed.
Q. Is the heat of solution of LiCl exothermic or endothermic?
The heat of the solution of LiCl is exothermic. When lithium and chloride ionize in water, they must first break apart from one another.
Q. Is calcium chloride exothermic or endothermic?
The dissolution of calcium chloride is an exothermic process.
Q. Is nac2h3o2 endothermic or exothermic?
When a seed crystal of sodium acetate triacetate is added, sodium acetate trihydrate crystallizes out. The heat of solution of sodium acetate trihydrate is 19.7 kJ/mole (an endothermic process). The crystallization is exothermic.
Q. Is calcium and water endothermic or exothermic?
The formation of slaked lime (calcium hydroxide, Ca(OH)2) when water is added to lime (CaO) is exothermic. CaO(s) + H2O (l) → Ca(OH)2(s) This reaction occurs when water is added to dry portland cement to make concrete, and heat evolution of energy as heat is evident because the mixture becomes warm.