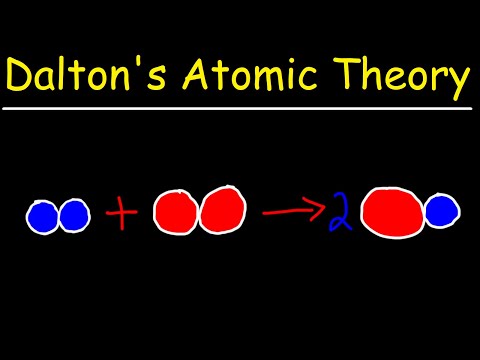
For more on isotopes, you can watch this video on atomic number, mass number, and isotopes. Despite these caveats, Dalton’s atomic theory is still mostly true, and it forms the framework of modern chemistry. Scientists have even developed the technology to see the world on an atomic level!
Q. What did Aristotle believe about the atom?
Aristotle did not believe in the atomic theory and he taught so otherwise. He thought that all materials on Earth were not made of atoms, but of the four elements, Earth, Fire, Water, and Air. He believed all substances were made of small amounts of these four elements of matter.
Table of Contents
- Q. What did Aristotle believe about the atom?
- Q. Who is the philosopher of atom?
- Q. Why did Democritus believe in atoms?
- Q. What did he believe about atoms?
- Q. Who is the father of Proton?
- Q. Who is the father of electron?
- Q. What was Chadwick’s experiment called?
- Q. What did Chadwick prove?
- Q. How did James Chadwick prove the existence of neutrons?
- Q. Who is discovered nucleus?
- Q. What is the charge of a neutron?
- Q. What is the charge of a neutron positive or negative?
- Q. What is a neutron made of?
- Q. How do you shoot a neutron?
- Q. How do you represent neutrons?
- Q. Which atom has the largest number of neutrons?
- Q. What is the symbol of a proton?
- Q. Are protons equal to neutrons?
- Q. Who discovered Proton?
Q. Who is the philosopher of atom?
Leucippus of Miletus (5th century bce) is thought to have originated the atomic philosophy. His famous disciple, Democritus of Abdera, named the building blocks of matter atomos, meaning literally “indivisible,” about 430 bce.
Q. Why did Democritus believe in atoms?
Democritus thought that different kinds of matter vary because of the size, shape, and arrangement of their atoms. Differences in the weight of matter, he argued, could be explained by the closeness of atoms. Atoms of lighter matter, he thought, were more spread out and separated by more empty space.
Q. What did he believe about atoms?
In the 5th century BCE, Leucippus and his pupil Democritus proposed that all matter was composed of small indivisible particles called atoms. Democritus believed that atoms are too small for human senses to detect, they are infinitely many, they come in infinitely many varieties, and that they have always existed.
Q. Who is the father of Proton?
Ernest Rutherford
Q. Who is the father of electron?
Thomson
Q. What was Chadwick’s experiment called?
Explanation: According to PhysicsLab Online, James Chadwick was assigned the task of tracking down evidence of Rutherford’s tightly bound, but theoretical, “proton-electron pair.” Chadwick’s experiment showed this was actually a different subatomic particle, now called the neutron.
Q. What did Chadwick prove?
In 1932, Chadwick made a fundamental discovery in the domain of nuclear science: he proved the existence of neutrons – elementary particles devoid of any electrical charge. For this epoch-making discovery he was awarded the Hughes Medal of the Royal Society in 1932, and subsequently the Nobel Prize for Physics in 1935.
Q. How did James Chadwick prove the existence of neutrons?
The only good explanation for his result was a neutral particle. To prove that the particle was indeed the neutron, Chadwick measured its mass. For his mass measurement, Chadwick bombarded boron with alpha particles. Like beryllium, boron emitted neutral rays.
Q. Who is discovered nucleus?
Ernest Rutherford’s
Q. What is the charge of a neutron?
Neutron, neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1,839 times greater than that of the electron.
Q. What is the charge of a neutron positive or negative?
Proton—positive; electron—negative; neutron—no charge. The charge on the proton and electron are exactly the same size but opposite. The same number of protons and electrons exactly cancel one another in a neutral atom.
Q. What is a neutron made of?
A neutron is made of 3 quarks, one up quark, and 2 down quarks and many many “intermediate particles” called gluons which carry the interaction between the quarks. These gluons are exchanged very often, so the quarks feel each of other. Neutrons do not always decay.
Q. How do you shoot a neutron?
With U-238, the neutron needs to be slowed down, or “thermalized”. This is done using heavy water, or water made from hydrogen with extra neutrons. The free neutrons hit the water atoms and transfer some of their kinetic energy to the water, heating it up.
Q. How do you represent neutrons?
The neutron number, symbol N, is the number of neutrons in a nuclide. Atomic number (proton number) plus neutron number equals mass number: Z + N = A.
Q. Which atom has the largest number of neutrons?
livermorium
Q. What is the symbol of a proton?
p+
Q. Are protons equal to neutrons?
The mass number of an atom is equal to the number of protons plus the number of neutrons that it contains. In other words, the number of neutrons in any atom is its mass number minus its atomic number….
| Atomic number | = number of protons per atom |
|---|---|
| = number of electrons per neutral atom |