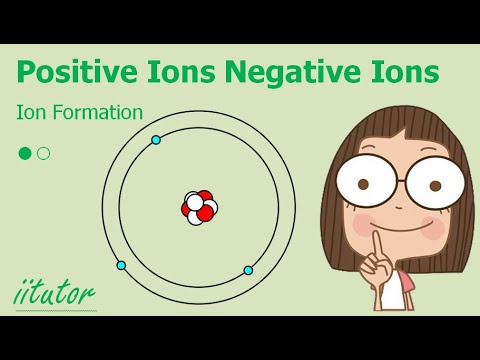
Atoms with a charge are known as IONS. More precisely atoms with a positive charge are known as cations while atoms with a negative charge are known as anions. Boron, above, forms a cation.
Q. How Ionic is BCl3?
BCl3 will have higher ionic character. This is attributed to the fact that it’s atomic number is 5 and electron distribution: 2,3 i.e. 2s2 2p1 , size of it is comparatively larger than carbon and hold on shared electrons is accordingly changes the electronegativity so as the ionic character.
Table of Contents
- Q. How Ionic is BCl3?
- Q. Is boron Trihydride ionic or covalent?
- Q. Is Boron molecular or ionic?
- Q. Can boron make ionic bonds?
- Q. What is the most common ion of boron?
- Q. What is the ion formula of boron?
- Q. What makes boron unique?
- Q. What is the ionic charge of aluminum?
- Q. What are H+ ions called?
- Q. What is the Ion name for magnesium?
- Q. Why does magnesium ion have a 2+ charge?
- Q. Why do ions in magnesium oxide stay together?
Q. Is boron Trihydride ionic or covalent?
Boron generally does not accept electrons to form a negative ion, so it does not normally form ionic compounds — the chemistry of boron is essentially covalent.
Q. Is Boron molecular or ionic?
Boron is capable of forming ionic and covalent bonds, but cannot form metallic bonds.
Q. Can boron make ionic bonds?
Boron is a metalloid, intermediate between metals and non-metals. It exists in many polymorphs (different crystal lattice structures), some more metallic than others. Metallic boron is extremely hard and has a very high melting point. Boron does not generally make ionic bonds, it forms stable covalent bonds.
Q. What is the most common ion of boron?
The most common and well-known boron hydrides include the decahydro-closo-decaborate ([B10H10]2−) and dodecahydro-closo-dodecaborate ([B12H12]2−) anions. When boron hydride clusters include carbon atoms, they form carboranes, or carbaboranes (according to International Union of Pure and Applied Chemistry nomenclature).
Q. What is the ion formula of boron?
Boron suboxide (chemical formula B6O) is a solid compound containing six boron atoms and one oxygen atom.
Q. What makes boron unique?
Boron, in its crystalline form, is the second-hardest element behind carbon (in its diamond form), according to Chemicool. Unlike many elements, which form in fusion reactions within stars, boron formed after the Big Bang by a process called cosmic ray spallation.
Q. What is the ionic charge of aluminum?
3+
Q. What are H+ ions called?
The hydrogen nucleus is made up of a particle carrying a unit positive electric charge, called a proton. The isolated hydrogen ion, represented by the symbol H+, is therefore customarily used to represent a proton.
Q. What is the Ion name for magnesium?
Magnesium(2+) is a magnesium cation, a divalent metal cation and a monoatomic dication. It has a role as a cofactor. Magnesium cation is a Calculi Dissolution Agent….4.3Related Element.
| Element Name | Magnesium |
|---|---|
| Element Symbol | Mg |
| Atomic Number | 12 |
Q. Why does magnesium ion have a 2+ charge?
Explanation: Magnesium is under group 2 that also has a valence electron of 2. To achieve stability and to follow the octet rule, these 2 electrons in the outer shell will be removed making this atom into an ion with a 2+ charge.
Q. Why do ions in magnesium oxide stay together?
Another example of ionic bonding takes place between magnesium (Mg) and oxygen (O) to form magnesium oxide (MgO). Magnesium loses two electrons to form Mg2+, and oxygen gains two electrons to form O2-. The attractive force between the oppositely charged ions is what holds the molecule together.