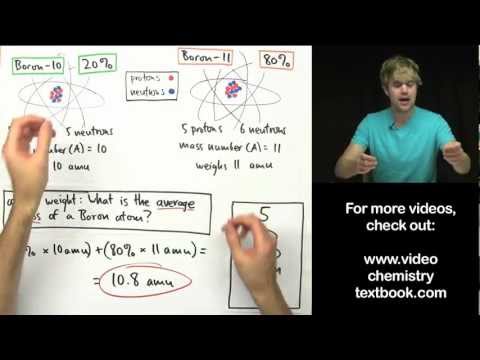
Atomic Mass Versus Atomic Weight Atomic mass (ma) is the mass of an atom. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists.
Q. Which elements have different number of protons and electrons?
All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons….Atomic Number.
Table of Contents
- Q. Which elements have different number of protons and electrons?
- Q. What is the difference between the number of protons and neutrons of the element?
- Q. What number is the atomic mass?
- Q. What is the one thing that determines the identity of an atom?
- Q. How could you tell what element it is if only a model was present?
- Q. What determines the identity of a beryllium atom?
- Q. How many electrons are in a beryllium atom?
- Q. What makes beryllium unique?
- Q. Is beryllium a neutral atom?
- Q. Why is beryllium used in aircraft?
- Q. Does the human body use beryllium?
- Q. Is beryllium safe to touch?
- Q. What metals contain beryllium?
- Q. Where is beryllium used in everyday life?
- Q. What foods contain beryllium?
| Name | Helium |
|---|---|
| Neutrons | 2 |
| Electrons | 2 |
| Atomic Number (Z) | 2 |
| 4 |
Q. What is the difference between the number of protons and neutrons of the element?
The number of protons determines an element’s atomic number and is used to distinguish one element from another. The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess.
Q. What number is the atomic mass?
Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2+2=4, we know that the mass number of the helium atom is 4….Mass Number.
| Name | helium |
|---|---|
| Symbol | He |
| Atomic Number (Z) | 2 |
| Protons | 2 |
| Neutrons | 2 |
Q. What is the one thing that determines the identity of an atom?
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.
Q. How could you tell what element it is if only a model was present?
Correct answer: There are two properties that can be used to identify an element: the atomic number or the number of protons in an atom. The number of neutrons and number of electrons are frequently equal to the number of protons, but can vary depending on the atom in question.
Q. What determines the identity of a beryllium atom?
The number of protons in one atom of an element determines the atom’s identity, and the number of electrons determines its electrical charge. The atomic number tells you the number of protons in one atom of an element.
Q. How many electrons are in a beryllium atom?
2,2
Q. What makes beryllium unique?
Uniquely strong and light, beryllium is used to make cell phones, missiles and aircrafts. Steel gray in color, beryllium’s modulus of elasticity is about one-third greater than steel. Beryllium is nonmagnetic and resistant to concentrated nitric acid.
Q. Is beryllium a neutral atom?
This means that an atom of hydrogen has one proton, and, if it’s neutral, one electron as well….Atomic Number.
| Name | Beryllium |
|---|---|
| Protons | 4 |
| Neutrons | 5 |
| Electrons | 4 |
| Atomic Number (Z) | 4 |
Q. Why is beryllium used in aircraft?
Beryllium alloys are used in automobile components and airplane equipment to ensure the reliable operation of vital equipment and to enhance fuel efficiency. These weight savings, in turn, make planes more fuel efficient which results in reduced exhaust emissions.
Q. Does the human body use beryllium?
Most likely, once in the body, beryllium combines with certain proteins, causing the release of toxic substances. These are responsible for the lesions seen in the lungs. Certain cells form masses of tissue called granulomas in response to beryllium.
Q. Is beryllium safe to touch?
* Beryllium Oxide is a CARCINOGEN–HANDLE WITH EXTREME CAUTION. * Contact can cause eye irritation, redness, itching and burning. * Beryllium Oxide can irritate and burn the skin. Higher exposure may cause skin ulcers to develop.
Q. What metals contain beryllium?
It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl (aquamarine, emerald) and chrysoberyl. As a free element it is a steel-gray, strong, lightweight and brittle alkaline earth metal.
Q. Where is beryllium used in everyday life?
Beryllium is used in gears and cogs particularly in the aviation industry. Beryllium is a silvery-white metal. It is relatively soft and has a low density. Beryllium is used in alloys with copper or nickel to make gyroscopes, springs, electrical contacts, spot-welding electrodes and non-sparking tools.
Q. What foods contain beryllium?
However, some fruits and vegetables such as kidney beans and pears may contain significant levels of beryllium. These levels can enter animals that eat them, but luckily most animals excrete beryllium quickly through urine and feces. The uptake of beryllium has consequences mainly for human health.