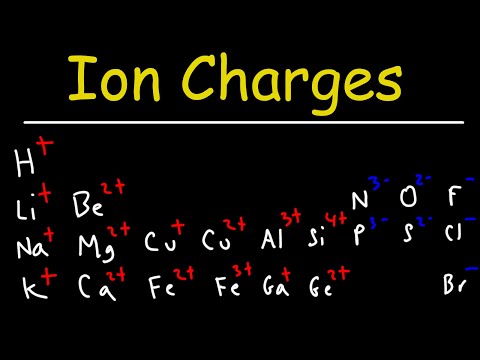Most Group 3 metals (aluminum, gallium, and indium) form 3+ cations. The cation of aluminum, thus, is designated as Al 3+ . Group 6 nonmetals and metalloids (oxygen, sulfur, selenium, and tellurium) form 2− anions. Oxygen, in its normal ionized state, is shown as O 2− .
Q. What is the ratio of ions?
The formula Mg 2Cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio….Looking Closer: Blood and Seawater.
Table of Contents
- Q. What is the ratio of ions?
- Q. What is the charge of an ion formed by aluminum?
- Q. Why does aluminum ion have a 3 charge?
- Q. What is the core charge of aluminum?
- Q. What is the charge for F?
- Q. How many electrons are in an ion of aluminum?
- Q. How do you balance a chemical equation Vedantu?
- Q. What is a chemical equation Why is it necessary to balance it?
- Q. What is chemical equation Why should it be balanced?
| Ion | Percent in Seawater | Percent in Blood |
|---|---|---|
| Na + | 2.36 | 0.322 |
| Cl − | 1.94 | 0.366 |
| Mg 2 + | 0.13 | 0.002 |
| SO 4 2− | 0.09 | — |
Q. What is the charge of an ion formed by aluminum?
The charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons.
Q. Why does aluminum ion have a 3 charge?
What is the charge of aluminum ion? As Al has 3 valence electrons as M(3) so it tends to lose electrons then after losing these 3 electrons it has 10 electrons and 13 protons, and 10 electrons are neutralized by 10 protons out of 13, and the further excess 3 protons out of 13 appear as +3 charge.
Q. What is the core charge of aluminum?
Also, what is the core charge of aluminum? As Al has 3 valence electrons as M(3) so it tends to lose electrons then after losing these 3 electrons it has 10 electrons and 13 protons, and 10 electrons are neutralized by 10 protons out of 13, and the further excess 3 protons out of 13 appear as +3 charge.
Q. What is the charge for F?
Table of Common Element Charges
| Number | Element | Charge |
|---|---|---|
| 9 | fluorine | 1- |
| 10 | neon | 0 |
| 11 | sodium | 1+ |
| 12 | magnesium | 2+ |
Q. How many electrons are in an ion of aluminum?
10 electrons
Q. How do you balance a chemical equation Vedantu?
If there are no inequalities found, the chemical equation is said to be balanced. In this example, now every element has an equal number of atoms in both reactant and product sides. Therefore, the balanced chemical equation becomes C3H8 + 5O2 → 3CO2 + 4H2O.
Q. What is a chemical equation Why is it necessary to balance it?
A chemical equation should always be balanced because the law of conservation of mass states that matter can neither be created nor destroyed so in a chemical equation the total mass of reactants must be equal to the mass of products formed i.e. the total number of atoms of each element should be equal on both the …
Q. What is chemical equation Why should it be balanced?
According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules present on the reactant side should be equal to the total mass of elements or molecules present on the product side.