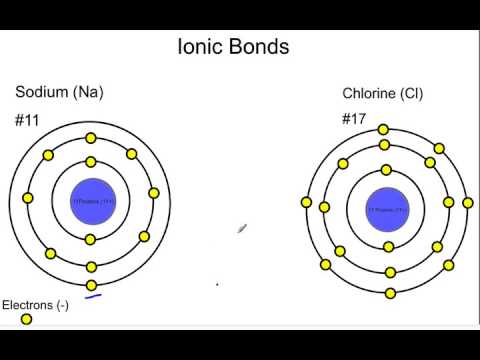
They combine as atoms, and separate as ions. When sodium and chlorine atoms come together to form sodium chloride (NaCl), they transfer an electron. Because the sodium ion has a positive charge, and the chlorine ion has a negative charge, they are attracted to each other, and form an ionic bond.
Q. Which element will bond ionically?
All transition metals and rare earth metals act as positives in ionic bonding. Hydrogen can be involved in ionic bonding. It will act as a nonmetal with anegative one charge. It is named hydride.
Table of Contents
- Q. Which element will bond ionically?
- Q. Which elements will bond ionically with chlorine?
- Q. What type of bond is B and BR?
- Q. How does calcium bond with chlorine?
- Q. What type of bond is AlF3?
- Q. What two types of bonds can calcium form?
- Q. What happens to chlorine in an ionic bond?
- Q. What is the rule of zero charge?
- Q. What is the ionic formula for a bond between calcium and nitrogen?
- Q. What is the formula of the compound formed by calcium and nitrogen?
- Q. What is the correct formula for the compound ca2+ and NO2?
- Q. What is the formula for aluminum and nitrogen?
- Q. What happens when Aluminium reacts with nitrogen according to the equation?
- Q. What is the chemical name for Ain?
- Q. What will be produced when aluminum and nitrogen react?
- Q. How many grams of NH3 are made from 50.0 g of nitrogen?
- Q. Does Ga react with nitrogen?
- Q. Does nitrogen gas react with anything?
- Q. What other elements does nitrogen mix with?
- Q. Is nitrogen a corrosive gas?
- Q. Is nitrogen a cold gas?
- Q. How much nitrogen is in a cylinder?
Q. Which elements will bond ionically with chlorine?
This type of chemical bond is called an ionic bond because the bond formed between two ions of opposite charge. The sodium cation (Na+) and the chlorine anion (Cl-) are attracted to one another to form sodium chloride, or table salt.
Q. What type of bond is B and BR?
BBr Bond Polarity
| Electronegativity (B) | 2.0 |
|---|---|
| Electronegativity (Br) | 3.0 |
| Electronegativity Difference | 1 Non-Polar Covalent = 0 0 < Polar Covalent < 2 Ionic (Non-Covalent) ≥ 2 |
| Bond Type | Polar Covalent |
| Bond Length | 1.888 angstroms |
Q. How does calcium bond with chlorine?
Two atoms of Chlorine and one atom of Calcium are near each other. Because of the stronger attraction, each chlorine pulls in one electron from the Calcium atom. The calcium became a positive charge atom, while the chlorine negative charge. From this, they attract each other and form an ionic bond.
Q. What type of bond is AlF3?
ionic
Q. What two types of bonds can calcium form?
Because the bond forms between a metal (Ca) and a non-metal (O), the bond will most likely be ionic. If a bond forms between two non-metals, then the bond is most likely covalent.
Q. What happens to chlorine in an ionic bond?
Chlorine gains an electron, leaving it with 17 protons and 18 electrons. Since it has 1 more electron than protons, chlorine has a charge of −1, making it a negative ion. When ions form, atoms gain or lose electrons until their outer energy level is full.
Q. What is the rule of zero charge?
The nonmetal atoms accept electrons and form ions with a negative charge, called anions. When an ionic compound forms, the total charge on the atoms adds up to zero. This is known as the rule of zero charge.
Q. What is the ionic formula for a bond between calcium and nitrogen?
Calcium nitride is a red-brown, crystalline solid made up of calcium and nitrogen. Its chemical formula is Ca3N2 . The ratio will be determined by the charges of the ions. The net charge of the compound will be zero.
Q. What is the formula of the compound formed by calcium and nitrogen?
Calcium nitride is the inorganic compound with the chemical formula Ca3N2.
Q. What is the correct formula for the compound ca2+ and NO2?
Calcium nitrite | Ca(NO2)2 – PubChem.
Q. What is the formula for aluminum and nitrogen?
AlN
Q. What happens when Aluminium reacts with nitrogen according to the equation?
Chemical Equation Balancer AL + N2 = ALN. Aluminium react with nitrogen to produce aluminium nitride(Aln). This reaction takes place at a temperature of 800-1200°C. Aluminium has a higher affinity for oxygen than nitrogen, and so in the presence of oxygen and nitrogen will form Al 2O 3 in preference to AlN.
Q. What is the chemical name for Ain?
AIN : Summary
| Code | AIN |
|---|---|
| Synonyms | ACETYLSALICYLIC ACID ASPIRIN |
| Systematic names | Program Version Name ACDLabs 10.04 2-(acetyloxy)benzoic acid OpenEye OEToolkits 1.5.0 2-acetyloxybenzoic acid |
| Formula | C9 H8 O4 |
| Formal charge | 0 |
Q. What will be produced when aluminum and nitrogen react?
Aluminum react with nitrogen to produce aluminum nitride.
Q. How many grams of NH3 are made from 50.0 g of nitrogen?
We have 50 g of nitrogen gas here, so we need to find the number of moles of nitrogen. From here, we would produce 1.79⋅2=3.58 moles of ammonia. 3.58mol ⋅17.031 gmol ≈61 g of NH3 .
Q. Does Ga react with nitrogen?
This is due to inert pair effect. On going down the group 13 elements, stability of +3 oxidation state decreases and +1 oxidation state increases.
Q. Does nitrogen gas react with anything?
Nitrogen does not react with other elements very well, even when they get hot. The filament can get very hot, but the metal of which it is made will not combine with nitrogen gas. The nitrogen gas is an inert atmosphere for the bulb. Another use for inert atmospheres is in protecting historic documents.
Q. What other elements does nitrogen mix with?
When nitrogen is heated, it combines directly with magnesium, lithium, or calcium. When mixed with oxygen and subjected to electric sparks, it forms nitric oxide (NO) and then the dioxide (NO2). When heated under pressure with hydrogen in the presence of a suitable catalyst , ammonia forms (Haber process).
Q. Is nitrogen a corrosive gas?
Liquid nitrogen is inert, colorless, odorless, non corrosive, nonflammable, and extremely cold. Nitrogen makes up the major portion of the atmosphere (78% by volume). Nitrogen is inert and will not support combustion; however, it is not life supporting.
Q. Is nitrogen a cold gas?
Liquid nitrogen is so cold because of the way molecules change as a gas turns to liquid. Nitrogen doesn’t naturally occur in a liquid form here on Earth. This compression causes the gas to heat up. While keeping the pressure high we cool it down to the temperature of the lab.
Q. How much nitrogen is in a cylinder?
A typical cylinder, about 5′ tall, can hold about 230 cubic feet of nitrogen gas, if it is filled to the maximum operating pressure – which can be in the range of 2,200 psi, that is to say, if it is full. The cylinder will have the maximum allowable pressure stamped on the side near the valve.