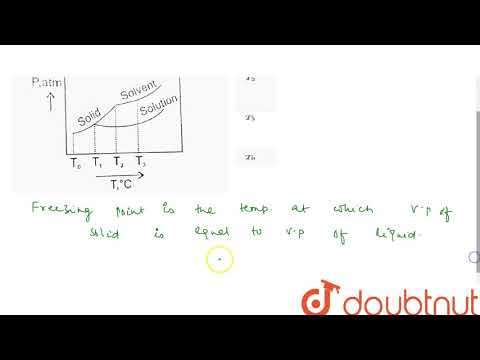
Freezing Point Depression:
Q. What is the normal freezing point of water?
32 °F.
Table of Contents
Q. What is the normal freezing?
The normal freezing point is the temperature at which a substance melts (or freezes) at one atmosphere (760 torr = 760 mm Hg = 14.7 psi = 101.3 kPa) of pressure.
- A solute lowers the freezing point of a solvent.
- In dilute solutions, the freezing point depression is proportional to the molality of the solute particles: ΔTf = -Kfm. ΔTf = the amount by which the freezing point is lowered.
- Freezing Point of solution = normal freezing point of solvent + ΔTf
Q. What is the normal point of water?
There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 °C (211.9 °F) at a pressure of 1 atm (i.e., 101.325 kPa). The IUPAC recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 °C (211.3 °F).
Q. Do all liquids boil at 100 degrees?
All liquids boil at 100 degrees Celsius. False. Different liquids have different boiling points. And liquid steel doesn’t boil until it’s heated to 3000°C.
Q. What liquid boils at 78 degrees?
Boiling temperatures for some common liquids and gases – acetone, butane, propane ..
| Product | Boiling Point at Atmospheric Pressure (oC) |
|---|---|
| Acrylonitrile | 77.2 |
| Alcohol – ethyl (grain, ethanol) C2H5OH | 79 |
| Alcohol – allyl | 97.2 |
| Alcohol – butyl-n | 117 |
Q. How do you cook inside without electricity?
6 Methods to Cook Indoors Without Electricity
- Tea Lite Oven.
- Chafing Pans & Chafing Gel Fuel.
- Crockpot & Solar Panels.
- Flameless Cooking System. The Barocook Flameless Cooking System is a good item to have in a power outage, on a road trip, or in an emergency kit.
- Fireplace.
- Portable Heaters, Safe or Not Safe? You Decide!