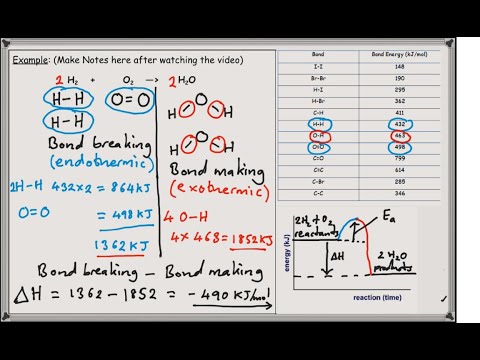
To calculate an energy change for a reaction:
Q. Where is the chemical energy stored?
Chemical energy is stored in the bonds that connect atoms with other atoms and molecules with other molecules. Because chemical energy is stored, it is a form of potential energy. When a chemical reaction takes place, the stored chemical energy is released.
Table of Contents
- Q. Where is the chemical energy stored?
- Q. What are the 3 sources of chemical energy?
- Q. How do you calculate total energy absorbed?
- Q. What is the formula you can use to calculate thermal energy changes?
- Q. What is the change in thermal energy of the gas?
- Q. What does Q Mcδt mean?
- Q. What is h in heat transfer?
- Q. What is the relationship between CP and CV?
Q. What are the 3 sources of chemical energy?
Chemical energy is energy stored in the bonds of atoms and molecules. Batteries, biomass, petroleum, natural gas, and coal are examples of chemical energy. Chemical energy is converted to thermal energy when people burn wood in a fireplace or burn gasoline in a car’s engine.
- add together the bond energies for all the bonds in the reactants – this is the ‘energy in’
- add together the bond energies for all the bonds in the products – this is the ‘energy out’
- energy change = energy in – energy out.
Q. How do you calculate total energy absorbed?
You can do this easily: just multiply the heat capacity of the substance you’re heating by the mass of the substance and the change in temperature to find the heat absorbed.
Q. What is the formula you can use to calculate thermal energy changes?
The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °C. The formula is Cv = Q / (ΔT ⨉ m) .
Q. What is the change in thermal energy of the gas?
An increase in temperature means that there’s an increase in the kinetic energy of the individual atoms. Gases are especially affected by thermal expansion, although liquids expand to a lesser extent with similar increases in temperature, and even solids have minor expansions at higher temperatures.
Q. What does Q Mcδt mean?
Heat Transfer
Q. What is h in heat transfer?
The convective heat transfer coefficient (h), defines, in part, the heat transfer due to convection. The convective heat transfer coefficient is sometimes referred to as a film coefficient and represents the thermal resistance of a relatively stagnant layer of fluid between a heat transfer surface and the fluid medium.
Q. What is the relationship between CP and CV?
The specific heat of gas at constant volume in terms of degree of freedom ‘f’ is given as: Cv = (f/2) R. So, we can also say that, Cp/Cv = (1 + 2/f), where f is degree of freedom. Monoatomic gas has only one translational motion, hence three translational degrees of freedom.