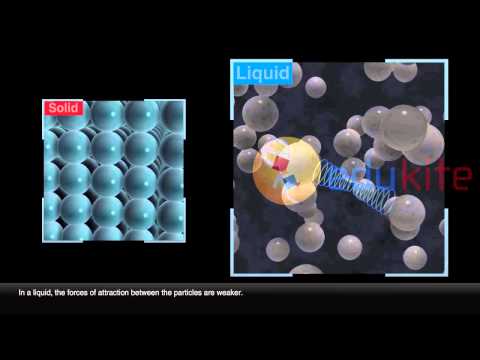
In gases the particles move rapidly in all directions, frequently colliding with each other and the side of the container. With an increase in temperature, the particles gain kinetic energy and move faster. In liquids, particles are quite close together and move with random motion throughout the container.
Q. How fast do molecules move in a gas?
As a side note, gas molecules tend to move very fast. At 0 °C the average H2 molecule is moving at about 2000 m/s, which is more than a mile per second and the average O2 molecule is moving at approximately 500 m/s.
Table of Contents
- Q. How fast do molecules move in a gas?
- Q. Do molecules speed up when heated?
- Q. In which state of matter are molecules moving the fastest?
- Q. How do molecules move in a solid?
- Q. How do water molecules move?
- Q. What are 3 characteristics of a solid?
- Q. How do molecules move in each state of matter?
- Q. What is the 3 states of matter?
- Q. Is a pumpkin a solid liquid or gas?
- Q. Can a solid flow?
- Q. What substance is solid but can flow?
- Q. Is Jelly solid or liquid?
- Q. Can water be compressed?
- Q. Why is liquid incompressible?
- Q. What happens when a fluid is compressed?
- Q. What Cannot be compressed?
- Q. Are any liquids compressible?
- Q. What are the 3 properties of a liquid?
- Q. What are three examples of liquids?
Q. Do molecules speed up when heated?
When heat is added to a substance, the molecules and atoms vibrate faster. As atoms vibrate faster, the space between atoms increases. The motion and spacing of the particles determines the state of matter of the substance. The end result of increased molecular motion is that the object expands and takes up more space.
Q. In which state of matter are molecules moving the fastest?
gases
Q. How do molecules move in a solid?
Solid – In a solid, the attractive forces keep the particles together tightly enough so that the particles do not move past each other. In the solid the particles vibrate in place. Liquid – In a liquid, particles will flow or glide over one another, but stay toward the bottom of the container.
Q. How do water molecules move?
Large quantities of water molecules constantly move across cell membranes by simple diffusion, often facilitated by movement through membrane proteins, including aquaporins. There are, however, many cases in which net flow of water occurs across cell membranes and sheets of cells.
Q. What are 3 characteristics of a solid?
They are:
- Definite mass, volume, and shape.
- Short Intermolecular distance.
- Strong Intermolecular Forces.
- The constituent particles remain fixed at their positions and can only oscillate about their mean positions.
- Solids are incompressible and rigid.
- High Density.
Q. How do molecules move in each state of matter?
gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other. solid vibrate (jiggle) but generally do not move from place to place.
Q. What is the 3 states of matter?
There are three very well known states of matter: Solids, Liquids, and Gases.
Q. Is a pumpkin a solid liquid or gas?
Pumpkin as a Solid We talked about the characteristics of a solid and then determined that it its natural state a pumpkin is a solid.
Q. Can a solid flow?
Because the particles don’t move, solids have a definite shape and volume, and can’t flow. Because the particles are already packed closely together, solids can’t easily be compressed.
Q. What substance is solid but can flow?
Granular materials are solids that can flow and behave like liquids. Examples of granular materials include sand, coffee, nuts, even corn flakes. Scientists consider things like icebergs or asteroids to be large granular materials, due to how they behave.
Q. Is Jelly solid or liquid?
Jelly is composed of liquid (usually a fruit juice) held in suspension by a lattice of flavorless proteins (usually gelatin, collagen or pectin). It is a suspension, which is a solid in a liquid.
Q. Can water be compressed?
Water is essentially incompressible, especially under normal conditions. Yet, in industrial applications water can be tremendously compressed and used to do things like cut through metal. Being incompressible, water makes a handy and useful tool for people to do work (and have fun).
Q. Why is liquid incompressible?
Liquids are usually considered incompressible. The molecules are already close together, so it is difficult to compress them any more. Under very high pressures, liquids will actually compress, but not very much.
Q. What happens when a fluid is compressed?
A consequence of compressing a fluid is that the viscosity, that is the resistance of the fluid to flow, also increases as the density increases. This is because the atoms are forced closer together, and thus cannot slip by each other as easily as they can when the fluid is at atmospheric pressure.
Q. What Cannot be compressed?
Solids are held in fixed positions, they can only vibrate. Because the particles can not move, solids have a definite shape and volume, and can’t flow. Because the particles are already packed closely together, solids can’t easily be compressed.
Q. Are any liquids compressible?
All liquids are compressible even water. Their densities will change as pressure is exerted. Compressibility is the fractional change in volume per unit increase in pressure.
Q. What are the 3 properties of a liquid?
1 Answer
- Liquids are almost incompressible. In liquids molecules are pretty close to each other.
- Liquids have fixed volume but no fixed shape.
- Liquids flow from higher to lower level.
- Liquids have their boiling points above room temperature, under normal conditions.
Q. What are three examples of liquids?
Examples of Liquids
- Water.
- Milk.
- Blood.
- Urine.
- Gasoline.
- Mercury (an element)
- Bromine (an element)
- Wine.