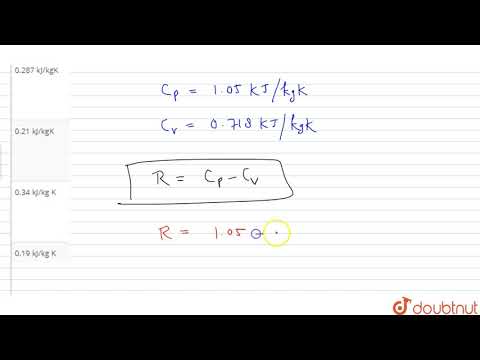The specific heat of dry air – CP and CV – will vary with pressure and temperature. This may influence on the accuracy of industrial air handling process calculations.
Q. What is relation between CP and CV?
The specific heat of gas at constant volume in terms of degree of freedom ‘f’ is given as: Cv = (f/2) R. So, we can also say that, Cp/Cv = (1 + 2/f), where f is degree of freedom. Monoatomic gas has only one translational motion, hence three translational degrees of freedom.
Table of Contents
- Q. What is relation between CP and CV?
- Q. How does CP change with pressure?
- Q. How many times faster than air does water conduct heat?
- Q. Does air transfer heat faster than water?
- Q. How could you show simply that water was a poor conductor of heat?
- Q. How can you prove that air is a bad conductor of heat?
- Q. How do you calculate CP from CV?
- Q. What is the ratio of CP CV for gas if the pressure?
- Q. Is CV constant with temperature?
- Q. What is the value of CV for Triatomic gas?
- Q. Is CV dependent on temperature?
- Q. Why does CP change with temperature?
- Q. Is CP dependent on pressure?
- Q. How does Gamma change with temperature?
- Q. How do you calculate gamma from pressure?
- Q. What is Gamma in isentropic process?
- Q. How do you calculate isentropic exponents?
- Q. Does isentropic mean adiabatic?
- Q. What is meant by isentropic flow?
- Q. Is density constant in isentropic flow?
- Q. Can shocks be present in isentropic flow?
Q. How does CP change with pressure?
Conventional thermodynamic expression predicts that the isobaric heat capacity decreases with increasing pressure. In model calculations, heat capacity increases with pressure, decreases, or remains insensitive to pressure, depending on the model applied.
Q. How many times faster than air does water conduct heat?
Water conducts heat away from your body 25 times faster than air does, so you cool much more rapidly in water. The human body’s internal temperature has to stay within a narrow range around 37° C (98.6° F) to function normally.
Q. Does air transfer heat faster than water?
Harvey Ramer said: Water will transfer heat 24.17 times faster than air. That is, and I quote from Engineering Toolbox “the quantity of heat transmitted through a unit thickness of a material – in a direction normal to a surface of unit area – due to a unit temperature gradient under steady state conditions”.
Q. How could you show simply that water was a poor conductor of heat?
Let us take some pure water in a test tube and dip a piece of wax in it after wrapping it in wire Then we hold the test tube in an inclined position and start heating water in the upper part of the tube we will observe that wax does not melt even when the upper part of water starts boiling Thus we can conclude that …
Q. How can you prove that air is a bad conductor of heat?
Take a hard glass test-tube and place in it small amount of wax. Heat the test-tube near the mouth. In few miniutes, the cork blows out, but wax does not melt. Thus, experiment proves that air is a bad conductor of heat.
Q. How do you calculate CP from CV?
cp = cv + R The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas.
Q. What is the ratio of CP CV for gas if the pressure?
The heat capacity at constant volume, Cv, is the derivative of the internal energy with respect to the temperature, so for our monoatomic gas, Cv = 3/2 R. The heat capacity at constant pressure can be estimated because the difference between the molar Cp and Cv is R; Cp – Cv = R.
Q. Is CV constant with temperature?
At ordinary temperatures, CV and CP increase only slowly as temperature increases. For many purposes they can be taken to be constant over rather wide temperature ranges. For real substances, CV is a weak function of volume, and CP is a weak function of pressure.
Q. What is the value of CV for Triatomic gas?
Three types of degrees of freedom are persisting, those being translational, rotational, and vibrational. This ratio γ=1.66 for an ideal monatomic gas and γ=1.4 for air, which is predominantly called as a diatomic gas.
Q. Is CV dependent on temperature?
The heat capacities of a substance increase with temperature. The rate of increase decreases as the temperature increases.
Q. Why does CP change with temperature?
As the substance heats up, the average kinetic energy of the molecules increases. The collisions impart enough energy to allow rotation to occur. Rotation then contributes to the internal energy and raises the specific heat.
Q. Is CP dependent on pressure?
Cp is (dH over dT) at constant pressure. Let’s start from enthalpy as a function of temperature and pressure. Then, the total differential of enthalpy is like this. So the temperature dependence of this function, dH over dP, gives pressure dependence of Cp.
Q. How does Gamma change with temperature?
Thus, the ratio of the two values, γ, decreases with increasing temperature. For more information on mechanisms for storing heat in gases, see the gas section of specific heat capacity.
Q. How do you calculate gamma from pressure?
gamma = cp / cv For air, gamma = 1.4 for standard day conditions. “Gamma” appears in several equations which relate pressure, temperature, and volume during a simple compression or expansion process. Because the value of “gamma” just depends on the state of the gas, there are tables of these values for given gases.
Q. What is Gamma in isentropic process?
During the compression process, as the pressure is increased from p1 to p2, the temperature increases from T1 to T2 according to this exponential equation. “Gamma” is just a number that depends on the gas. For air, at standard conditions, it is 1.4.
Q. How do you calculate isentropic exponents?
The isentropic exponent of a real gas is defined by (2) κ = − c p c v ( ∂ p ∂ v ) T ( v p ) , where and are the corresponding molar heat capacities of the gas at constant pressure and constant volume, while and are the pressure and specific volume.
Q. Does isentropic mean adiabatic?
An adiabatic is a process in which there is no heat transfer . This can be achieved by both reversibly and irreversible . The former one is called isentropic Process. An isentropic is an internally reversible adiabatic process which entropy of the system remains constant or Area and T-s diagram is zero .
Q. What is meant by isentropic flow?
In fluid dynamics, an isentropic flow is a fluid flow that is both adiabatic and reversible. That is, no heat is added to the flow, and no energy transformations occur due to friction or dissipative effects.
Q. Is density constant in isentropic flow?
Isentropic Flow Equations. As a gas is forced through a tube, the gas molecules are deflected by the walls of the tube. If the speed of the gas is much less than the speed of sound of the gas, the density of the gas remains constant and the velocity of the flow increases.
Q. Can shocks be present in isentropic flow?
Heat conduction from fluid element to fluid element Secondly, the isentropic flow also can break down through the appearance of shock waves. On the other hand, shock waves can appear only in supersonic flow; thus, if the speed of the fluidis everywhere subsonic, there is no danger of the compressibility burble.