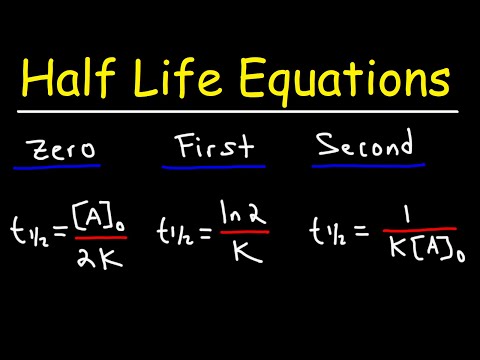
Since the half-life equation of a first-order reaction does not include a reactant concentration term, it does not rely on the concentration of reactant present. In other words, a half-life is independent of concentration and remains constant throughout the duration of the reaction.
Q. What is the current half life of knowledge?
Modern estimates place the half-life of an engineering degree at between 2.5 and 5 years, requiring between 10 and 20 hours of study per week.
Table of Contents
Q. How do you explain the half life of a drug?
The elimination half-life of a drug is a pharmacokinetic parameter that is defined as the time it takes for the concentration of the drug in the plasma or the total amount in the body to be reduced by 50%. In other words, after one half-life, the concentration of the drug in the body will be half of the starting dose.
Q. Does the half life of a second order reaction depends on the initial concentration?
By definition, the half life of any reaction is the amount of time it takes to consume half of the starting material. For a second-order reaction, the half-life is inversely related to the initial concentration of the reactant (A).
Q. What is half-life of reaction?
The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction: t1/2 = 0.693/k. Radioactive decay reactions are first-order reactions.
Q. How do you find the rate constant of a second-order reaction from a graph?
The integrated rate law for the second-order reaction A → products is 1/[A]_t = kt + 1/[A]_0. Because this equation has the form y = mx + b, a plot of the inverse of [A] as a function of time yields a straight line. The rate constant for the reaction can be determined from the slope of the line, which is equal to k.