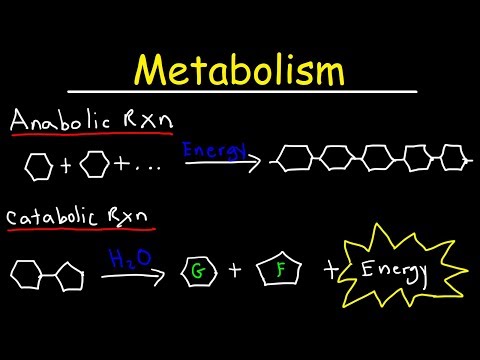
Two types of metabolic reactions take place in the cell: ‘building up’ (anabolism) and ‘breaking down’ (catabolism). Catabolic reactions give out energy. They are exergonic. In a catabolic reaction large molecules are broken down into smaller ones.
Q. Which reactions release energy anabolic or catabolic?
A cell’s metabolism refers to the combination of chemical reactions that take place within it. Catabolic reactions break down complex chemicals into simpler ones and are associated with energy release. Anabolic processes build complex molecules out of simpler ones and require energy.
Table of Contents
- Q. Which reactions release energy anabolic or catabolic?
- Q. What type of reaction is anabolism?
- Q. What are the final products of catabolic reactions?
- Q. What happens when an enzyme is involved in a catabolic reaction?
- Q. Are catabolic reactions reversible?
- Q. Are enzymes used up in a reaction?
- Q. What is meant by enzymes are not used up in a reaction?
- Q. How do enzymes control chemical reactions in the body?
- Q. What affects the enzyme rate of reaction?
- Q. What factors would decrease reaction rate?
- Q. How do enzymes affect a chemical reaction making it easier to occur?
- Q. Does pH affect enzyme inhibitors?
- Q. How does pH affect the rate of chemical reaction?
- Q. How and why do pH changes affect enzymes?
- Q. How does change in pH denature proteins?
Q. What type of reaction is anabolism?
Metabolism
| Type of metabolism | Process | Energetics |
|---|---|---|
| Anabolism | Builds complex molecules from simple ones | Endergonic |
| Catabolism | Breaks down complex molecules into simpler ones | Exergonic |
Q. What are the final products of catabolic reactions?
Pathways of AA Catabolism and Anabolism. Catabolism and anabolism of AA have some common characteristics. The same coenzymes are used in both paths. The final products of catabolic pathways and metabolic precursors of anabolic pathways are intermediates of glycolysis, citric acid cycle, and pentose phosphate pathway.
Q. What happens when an enzyme is involved in a catabolic reaction?
Enzymes control cellular reactions. As you remember, reactions that break down substances and release energy are called catabolic reactions. These reactions build larger, more complex, molecules from smaller ones. Photosynthesis and muscle growth from amino acids are examples of anabolic reactions.
Q. Are catabolic reactions reversible?
Note: This is a type of catabolic reaction (the larger glucose molecule is broken down to smaller carbon dioxide molecules) related to cellular energy production. In animal cells, such as humans, this is an irreversible reaction. Other metabolic reactions are called reversible reactions.
Q. Are enzymes used up in a reaction?
Enzymes aren’t changed or used up in the reactions they catalyze, so they can be used to speed up the same reaction over and over again. Each enzyme is highly specific for the particular reaction is catalyzes, so enzymes are very effective.
Q. What is meant by enzymes are not used up in a reaction?
Explanation: Enzymes can be thought as catalysts for metabolic reactions. Catalysts are not used up in reactions, as they do not participate in the actual reaction, but rather provide an alternate reaction pathway with a lower activation energy.
Q. How do enzymes control chemical reactions in the body?
Enzymes are biological catalysts. Catalysts lower the activation energy for reactions. The lower the activation energy for a reaction, the faster the rate. Thus enzymes speed up reactions by lowering activation energy.
Q. What affects the enzyme rate of reaction?
Several factors affect the rate at which enzymatic reactions proceed – temperature, pH, enzyme concentration, substrate concentration, and the presence of any inhibitors or activators.
Q. What factors would decrease reaction rate?
Lesson
- surface area of a solid reactant.
- concentration or pressure of a reactant.
- temperature.
- nature of the reactants.
- presence/absence of a catalyst.
Q. How do enzymes affect a chemical reaction making it easier to occur?
Explanation: Enzymes are catalysts which will lower the activation energy of a chemical reaction. By lowering the amount of energy needed to start a reaction, the reaction can go more quickly.
Q. Does pH affect enzyme inhibitors?
INHIBITION BY pH CHANGE pH has a marked effect on the velocity of enzyme-catalyzed reactions. The easiest assumption is that certain side chains necessary for catalysis must be in the correct protonation state.
Q. How does pH affect the rate of chemical reaction?
The rate of chemical reactions can be altered by changing pH, temperature, and/or the substrate concentration. Substrates are the compounds enzymes bond to. Optimal pH increases enzyme rate of reaction while less than optimal pH decreases it.
Q. How and why do pH changes affect enzymes?
pH: Each enzyme has an optimum pH range. Changing the pH outside of this range will slow enzyme activity. Extreme pH values can cause enzymes to denature. Enzyme concentration: Increasing enzyme concentration will speed up the reaction, as long as there is substrate available to bind to.
Q. How does change in pH denature proteins?
Changes in pH affect the chemistry of amino acid residues and can lead to denaturation. Protonation of the amino acid residues (when an acidic proton H + attaches to a lone pair of electrons on a nitrogen) changes whether or not they participate in hydrogen bonding, so a change in the pH can denature a protein.