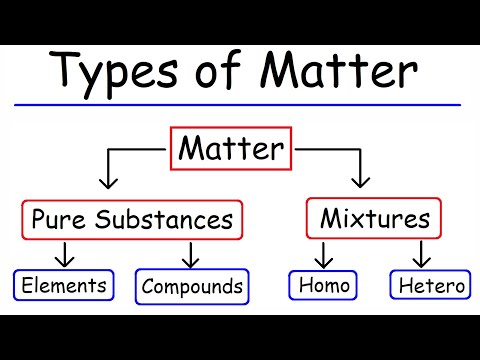
Elements are the simplest complete chemical substances. Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated back into their original components.
Q. Is salt water considered a mixture or a solution?
Salt water can be separated into its parts. You can let the water evaporate, and you will have just the salt left. Salt water is a solution because it has these two characteristics: it has the same concentration of each of its parts throughout the solution, and it can be separated by some physical process.
Table of Contents
- Q. Is salt water considered a mixture or a solution?
- Q. Why is salt water a mixture?
- Q. How do you describe a solution of salt and water?
- Q. How do you know if something is a mixture compound or element?
- Q. What are elements give 5 examples?
- Q. How many types of compounds are there?
- Q. What is the formula of water?
- Q. What is the formula for oxygen?
- Q. What is the name for H2O?
- Q. What is the chemical name for nbr3?
- Q. What is the chemical name for As2S5?
- Q. What is the chemical name of Ch?
- Q. Is Ch a chemical symbol?
- Q. What is the name of C2H4?
- Q. What is the chemical formula for C and O?
- Q. What is the formula of Tetraphosphorus Decoxide?
- Q. What is the chemical name of p2o5?
- Q. What is the chemical formula for C2H4?
- Q. Is ethylene harmful to humans?
- Q. Is ethylene good for humans?
- Q. How much ethylene does a banana produce?
Q. Why is salt water a mixture?
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. One characteristic of mixtures is that they can be separated into their components.
Q. How do you describe a solution of salt and water?
In a NaCl solution (salt-water), the solvent is water. A solute is the component in a solution in the lesser amount. In a NaCl solution, the salt is the solute. An aqueous solution is a solution in which water is the solvent.
Q. How do you know if something is a mixture compound or element?
If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture.
Q. What are elements give 5 examples?
Examples of elements include carbon, oxygen, hydrogen, gold, silver and iron.
Q. How many types of compounds are there?
There are four types of compounds, depending on how the constituent atoms are held together: molecules held together by covalent bonds. ionic compounds held together by ionic bonds.
Q. What is the formula of water?
H2O
Q. What is the formula for oxygen?
O2
Q. What is the name for H2O?
Oxidane
Q. What is the chemical name for nbr3?
Nitrogen tribromide Nitrogen
Q. What is the chemical name for As2S5?
Arsenic sulfide
Q. What is the chemical name of Ch?
Methylidyne radical
| Names | |
|---|---|
| show InChI | |
| show SMILES | |
| Properties | |
| Chemical formula | CH, CH•, CH3• |
Q. Is Ch a chemical symbol?
Thus, benzene is represented by the empirical formula CH, which indicates that a typical sample of the compound contains one atom of carbon (C) to one atom of hydrogen (H). Water is represented by the empirical formula H2O, denoting that the substance contains two atoms of hydrogen (H2) for every atom of oxygen (O).
Q. What is the name of C2H4?
Ethene
Q. What is the chemical formula for C and O?
Dicarbon monoxide
| Names | |
|---|---|
| show SMILES | |
| Properties | |
| Chemical formula | C2O |
| Molar mass | 40.021 g·mol−1 |
Q. What is the formula of Tetraphosphorus Decoxide?
P₂O₅
Q. What is the chemical name of p2o5?
tricyclo[3.3.1.13,7]tetraphosphoxane 1,3,5,7-tetraoxide
Q. What is the chemical formula for C2H4?
Q. Is ethylene harmful to humans?
* Exposure to Ethylene can cause headache, dizziness, fatigue, lightheadedness, confusion and unconsciousness. * Ethylene is a HIGHLY FLAMMABLE and REACTIVE chemical and a DANGEROUS FIRE and EXPLOSION HAZARD.
Q. Is ethylene good for humans?
Ethylene has been found not harmful or toxic to humans in the concentrations found in ripening rooms (100-150 ppm). In fact, ethylene was used medically as a anesthetic in concentrations significantly greater than that found in a ripening room.
Q. How much ethylene does a banana produce?
Around 2–50 µL ethylene·kg–1 fresh mate- rial·h–1 should be released during the initial ripening phases and can be measured with the method proposed.